Abstract
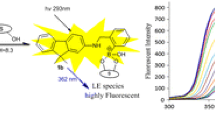
Carbohydrate sensing in an aqueous solution remains a very challenging area of interest. Using the idea of covalent reversible interaction between boronic acids and the diol groups in carbohydrates enable us to design a carbohydrate sensor 1-thianthrenylboronic acid (1T), which has high selectivity towards fructose. To elucidate the sensing and binding properties of 1T with sugars, we have incorporated theoretical (DFT and TD-DFT) and spectroscopic techniques. For an optimized geometry, the complete vibrational assignments were done with FT-IR and FT-Raman spectra. Physiochemical parameters were obtained by implementing frontier molecular orbital (FMO) analysis. Further, excited state properties were determined by performing TD-DFT calculations in solvent and these properties were in good agreement with the experiment. The steady state fluorescence measurements with varying concentration of sugars, revealed that the fluorescence intensity of boronic acid is enhanced by studied sugars due to the structural modification. We also noticed remarkable changes in fluorescence lifetimes and quantum yield after adding sugars. The article also reports influence of pH on boronic acid’s fluorescence intensity with and without sugars. The fluorescence of boronic acid increases with the increase in pH. These changes are due to acid–base equilibrium of boronic acid and led us to estimate the pKa value of 7.6. All the theoretical and experimental evidences suggested that 1T can be used as a possible fluorescent sensor for fructose. In addition, 1T showed very good affinity for Cu2+ ion with Ka = 150 × 102 M−1, which suggests that 1T can also be used as a chemosensor for Cu2+ ions.

















Similar content being viewed by others
References
Whyte GF, Vilar R, Woscholski R (2013) Molecular recognition with boronic acids-applications in chemical biology. J Chem Biol 6:161–174
Wu X, Chen XX, Jiang YB (2017) Recent advances in boronic acid-based optical chemosensors. Analyst 142:1403–1414
Silva MP, Saraiva L, Pinto M, Sousa ME (2020) Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules 25:4323
Pappin B, Kiefel MJ, Houston TA (2012) Boron-carbohydrate interactions. Carbohydrates-comprehensive studies on glycobiology and glycotechnology. Rijeka, Croatia
Zhai W, Sun X, James TD, Fossey JS (2015) Boronic acid-based carbohydrate sensing. Chemistry-An Asian Journal 10:1836–1848
Hansen JS, Christensen JB (2013) Recent advances in fluorescent arylboronic acids for glucose sensing. Biosensors 3:400–418
Lacina K, Skládal P, James TD (2014) Boronic acids for sensing and other applications-a mini-review of papers published in 2013. Chem Cent J 8:60
Yoon J, Czarnik AW (1992) Fluorescent chemosensors of carbohydrates. A means of chemically communicating the binding of polyols in water based on chelation-enhanced quenching. J Am Chem Soc 114:5874–5875
James TD, Sandanayake KS, Iguchi R, Shinkai S (1995) Novel saccharide-photoinduced electron transfer sensors based on the interaction of boronic acid and amine. J Am Chem Soc 117:8982–8987
DiCesare N, Lakowicz JR (2001) Evaluation of two synthetic glucose probes for fluorescence-lifetime-based sensing. Anal Biochem 294:154–160
Maity D, Hari N, Mohanta S (2017) A Bis (Boronic Ester) -Based Fluorogenic and Chromogenic Sensor for F– and Cu2+. ChemistrySelect 2:9037–9045
KyungáKwon S, NaáLee H (2008) Boronic acid-linked fluorescent and colorimetric probes for copper ions. Chem Commun 45:5915–5917
Li M, Ge H, Arrowsmith RL, Mirabello V, Botchway SW, Zhu W, Pascu SI, James TD (2014) Ditopic boronic acid and imine-based naphthalimide fluorescence sensor for copper (II). Chem Commun 50:11806–11809
Tharmaraj V, Pitchumani K (2013) D-Glucose sensing by (E)-(4-((pyren-1-ylmethylene) amino) phenyl) boronic acid via a photoinduced electron transfer (PET) mechanism. RSC Adv 3:11566–11570
Lathiotakis NN, Theodorakopoulos G, Petsalakis ID (2017) Electron transfer through organic molecular wires: A theoretical study. Chem Phys Lett 667:45–50
Bhavya NR, Mahendra M, Doreswamy BH, Kumar S, Gilandoust M, El-khatatneh NA (2019) Computational and spectroscopic investigations on boronic acid based fluorescent carbohydrate sensor in aqueous solution at physiological pH 7.5. J Mol Struct 1194:305–319
Khamees HA, Revanna BN, Madegowda M, Sebastian J, Haruvegowda DB, Kumar S (2020) Structural, Quantum Chemical and Spectroscopic Investigations on Photophysical Properties of Fluorescent Saccharide Sensor: Theoretical and Experimental Studies. ChemistrySelect 5:5227–5238
a|e - UV-Vis-IR Spectral Software 1.2, FluorTools, https://www.fluortools.com
Taniguchi M, Du H, Lindsey JS (2018) PhotochemCAD 3: diverse modules for photophysical calculations with multiple spectral databases. Photochem Photobiol 94:277–289
Bruker APEX (2004) SAINT-plus and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst Sect C 71:3–8
Frisch A (2009) Gaussian 09w reference. Wallingford, USA, p 25p
Tirado-Rives J, Jorgensen WL (2008) Performance of B3LYP density functional methods for a large set of organic molecules. J Chem Theory Comput 4:297–306
Jamroz MH (2004) Vibrational energy distribution analysis VEDA 4
Rudberg E, Sałek P, Helgaker T, Ågren H (2005) Calculations of two-photon charge-transfer excitations using Coulomb-attenuated density-functional theory. J Chem Phys 123:184108
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Iozzi MF, Mennucci B, Tomasi J, Cammi R (2004) Excitation energy transfer (EET) between molecules in condensed matter: A novel application of the polarizable continuum model (PCM). J Chem Phys 120:7029–7040
Mennucci B (2012) Polarizable continuum model. Wiley Interdisciplinary Reviews: Computational Molecular Science 2:386–404
Gutowski M, van Duijneveldt-van de Rijdt JG, van Lenthe JH, van Duijneveldt FB, (1993) Accuracy of the Boys and Bernardi function counterpoise method. J Chem Phys 98:4728–4737
Wolff SK, Grimwood DJ, McKinnon JJ, Jayatilaka D, Spackman MA (2007) Crystalexplorer 17.5; University of Western Australia, Perth
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Alver Ö (2011) DFT, FT-Raman, FT-IR, solution and solid-state NMR studies of 2, 4-dimethoxyphenylboronic acid. C R Chim 14:446–455
Alver Ö, Kaya MF (2014) Experimental and DFT studies on the vibrational spectra of 1H-indene-2-boronic acid. J Mol Struct 1076:147–152
Faniran JA, Shurvell HF (1968) Infrared spectra of phenylboronic acid (normal and deuterated) and diphenyl phenylboronate. Can J Chem 46:2089–2095
Zukerman-Schpector J, Madureira LS, Stefani HA, Gozhina O, Tiekink ER (2016) Structural systematics of aryl-1, 3-dithiane derivatives: crystal and energy-minimised structures, and Hirshfeld surface analysis. Zeitschrift für Kristallographie-Crystalline Materials 231:329–339
Parlak C, Ramasami P, Tursun M, Rhyman L, Kaya MF, Atar N, Alver Ö, Şenyel M (2015) 4-Mercaptophenylboronic acid: Conformation, FT-IR, Raman, OH stretching and theoretical studies. Spectrochim Acta Part A Mol Biomol Spectrosc 144:131–138
Karabacak M, Sinha L, Prasad O, Asiri AM, Cinar M (2013) An experimental and theoretical investigation of Acenaphthene-5-boronic acid: Conformational study, NBO and NLO analysis, molecular structure and FT-IR, FT-Raman, NMR and UV spectra. Spectrochim Acta Part A Mol Biomol Spectrosc 115:753–766
Fahim AM, Farag AM, Shaaban MR, Ragab EA (2018) Synthesis and DFT study of novel pyrazole, thiophene, 1, 3-thiazole and 1, 3, 4-thiadiazole derivatives. Eur J Chem 9:30–38
Karabacak M, Kose E, Atac A, Asiri AM, Kurt M (2014) Monomeric and dimeric structures analysis and spectroscopic characterization of 3, 5-difluorophenylboronic acid with experimental (FT-IR, FT-Raman, 1H and 13C NMR, UV) techniques and quantum chemical calculations. J Mol Struct 1058:79–96
Karabacak M, Kurt M (2008) Comparison of experimental and density functional study on the molecular structure, infrared and Raman spectra and vibrational assignments of 6-chloronicotinic acid. Spectrochim Acta Part A Mol Biomol Spectrosc 71:876–883
Dennington R, Keith T, Millam J (2009) GaussView, version 5
Stuart B (2004) Infrared spectroscopy: Fundamental and applications
Ayyappan S, Sundaraganesan N, Kurt M, Sertbakan TR, Özduran M (2010) Molecular structure, vibrational spectroscopic studies and NBO analysis of the 3, 5-dichlorophenylboronic acid molecule by the density functional method. J Raman Spectrosc 41:1379–1387
Karabacak M, Kose E, Atac A, Cipiloglu MA, Kurt M (2012) Molecular structure investigation and spectroscopic studies on 2, 3-difluorophenylboronic acid: A combined experimental and theoretical analysis. Spectrochim Acta Part A Mol Biomol Spectrosc 97:892–908
Erdogdu Y, Tahir Güllüoǧlu M, Kurt M (2009) DFT, FT-Raman, FT-IR and NMR studies of 2-fluorophenylboronic acid. Journal of Raman Spectroscopy: An International Journal for Original Work in all Aspects of Raman Spectroscopy, Including Higher Order Processes, and also Brillouin and Rayleigh Scattering 40:1615–1623
Alver Ö, Parlak C (2010) DFT, FT-Raman, FT-IR, liquid and solid-state NMR studies of 2, 6-dimethoxyphenylboronic acid. Vib Spectrosc 54:1–9
Dikmen G, Alver Ö (2015) NMR, FT-IR, Raman and UV–Vis spectroscopic investigation and DFT study of 6-Bromo-3-Pyridinyl Boronic Acid. J Mol Struct 1099:625–632
Alver Ö, Kaya MF, Dikmen G (2015) Structural characterization, solvent effects on nuclear magnetic shielding tensors, experimental and theoretical DFT studies on the vibrational and NMR spectra of 3-(acrylamido) phenylboronic acid. J Mol Struct 1102:285–294
Sas EB, Kurt M, Can M, Horzum N, Atac A (2016) Spectroscopic studies on 9H-carbazole-9-(4-phenyl) boronic acid pinacol ester by DFT method. J Mol Struct 1118:124–138
Rosso TE, Ellzy MW, Jensen JO, Hameka HF, Zeroka D (1998) Vibrational frequencies and structural determinations of 1, 4-dithiane. Spectrochim Acta Part A Mol Biomol Spectrosc 55:121–134
Gunasekaran S, Kumaresan S, Arunbalaji R, Anand G, Seshadri S, Muthu S (2009) Vibrational assignments and electronic structure calculations for 6-thioguanine. Journal of Raman Spectroscopy: An International Journal for Original Work in all Aspects of Raman Spectroscopy, Including Higher Order Processes, and also Brillouin and Rayleigh Scattering 40:1675–1681
Kurt M (2009) DFT simulations and Vibrational spectra of 4-chloro and 4-bromophenylboronic acid molecules. Journal of Raman Spectroscopy: An International Journal for Original Work in all Aspects of Raman Spectroscopy, Including Higher Order Processes, and also Brillouin and Rayleigh Scattering 40:67–75
Parr RG (1980) Density functional theory of atoms and molecules. In Horizons of quantum chemistry. Springer, Dordrecht
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis. CrystEngComm 11:19–32
Lipparini F, Barone V (2011) Polarizable force fields and polarizable continuum model: A fluctuating charges/PCM approach. 1. Theory and implementation. J Chem Theory Comput 7:3711–3724
Melavanki R, Sharma K, Yallur BC, Kusanur R, Sadasivuni KK, Singh D, Mane S, Katagi K, Pattar SV (2020) Understanding the binding interaction between phenyl boronic acid P1 and sugars: determination of association and dissociation constants using S-V plots, steady- state spectroscopic methods and molecular docking. Luminescence 36:163–168
Bhavya P, Melavanki R, Narayanappa CK, Kusanur R, U M, (2020) Binding interaction between boronic acid derivatives with monosaccharaides: effect of structural change of monosaccharaides upon binding using S-V plots. Macromol Symp 392:2000166
Yan J, Springsteen G, Deeter S, Wang B (2004) The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—it is not as simple as it appears. Tetrahedron 60:11205–11209
Taniguchi M, Lindsey JS (2018) Database of absorption and fluorescence spectra of> 300 common compounds for use in photochemCAD. Photochem Photobiol 94:290–327
Balabin RM (2010) Communications: Intramolecular basis set superposition error as a measure of basis set incompleteness: Can one reach the basis set limit without extrapolation?. J Chem Phys 132:211103
Gutowski M, van Duijneveldt-van de Rijdt JG, van Lenthe JH, van Duijneveldt F. B, (1993) Accuracy of the Boys and Bernardi function counterpoise method. J Chem Phys 98:4728–4737
Acknowledgements
The author would like to thank “Karnataka Science and Technology Promotion Society (K-STePS), Department of Science and Technology (DST) and Government of Karnataka” for providing financial support to carry out this research. The authors would like to thank Sophisticated Analytical Instrumentation facility (SAIF), Indian Institute of Technology, Madras, for providing FT-IR, FT-Raman spectra and single crystal X-ray diffraction data. And also, University with Potential for Excellence (UPE) University of Mysore for providing, fluorescence and UV-absorption spectrometer facility. We would like to thank Dr. Jeyaseelan S., for providing computational facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Revanna, B.N., Madegowda, M. Dithiane Based Boronic Acid as a Carbohydrate Sensor in an Aqueous Solution at pH 7.5: Theoretical and Experimental Approach. J Fluoresc 31, 1683–1703 (2021). https://doi.org/10.1007/s10895-021-02791-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02791-4