Abstract
Fluorescein molecules are extensively used to develop fluorescent probes for various analytes due to their excellent photophysical properties and the spirocyclic structure. The main structural modification of fluorescein occurs at the carboxyl group where different groups can be easily introduced to produce the spirolactam structure which is non-fluorescent. The spirolactam ring opening accounts for the fluorescence and the dual sensing of analytes using fluorescent sensors is still a topic of high interest. There is an increase in the number of dual sensors developed in the past five years and quite a good number of fluorescein derivatives were also reported based on reversible mechanisms. This review analyses environmentally and biologically important cations such as Cu2+, Hg2+, Fe3+, Pd2+, Zn2+, Cd2+, and Mg2+; anions (F−, OCl−) and small molecules (thiols, CO and H2S). Structural modifications, binding mechanisms, different strategies and a comparative study for selected cations, anions and molecules are outlined in the article.




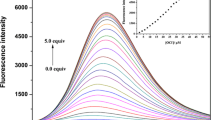
Reproduced with permission from Ref. [55]. Copyright (2016) The Royal Society of Chemistry."

Reproduced with permission from Ref [75], Copyright (2017) Elsevier."

Reproduced with permission from Ref [84], Copyright (2019) The Royal Society of Chemistry."

Reproduced with permission from Ref [116], Copyright (2016) Elsevier."

Reproduced with permission from Ref [122], Copyright (2015) Wiley."

Reproduced with permission from Ref [128], Copyright (2017) Elsevier”

Reproduced with permission from Ref [130], Copyright (2017) Elsevier.”

Reproduced with permission from Ref [136], Copyright (2016) The Royal Society of Chemistry."

Reproduced with permission from Ref [141], Copyright (2015) Elsevier”

Reproduced with permission from Ref [147], Copyright (2012) Elsevier”

Reproduced with permission from Ref. [170], Copyright (2016) Elsevier”

Reproduced with permission from Ref. [187], Copyright (2016) Elsevier”

Reproduced with permission from Ref. [193] Copyright (2017) American Chemical Society”

Reproduced with permission from Ref. [204] Copyright (2017) American Chemical Society”
Similar content being viewed by others
References
Nagarkar SS, Joarder B, Chaudhari AK, Mukherjee S, Ghosh SK (2013) Highly selective detection of nitro explosives by a luminescent metal-organic framework. Angew Chem Int Ed 52:2881–2885. https://doi.org/10.1002/anie.201208885
Fallas MM, Tanaka N, Buckenmaier SMC, McCalley DV, Chromator J (2013) Influence of phase type and solute structure on changes in retention with pressure in reversed-phase high performance liquid chromatography. J Chromatogr A 1297:37–45. https://doi.org/10.1016/j.chroma.2013.04.006
Rijo R, Riya D, AnithaV SYN, Louis G (2021) Recent advances in bimetallic based nanostructures: Synthesis and electrochemical sensing applications. Microchem J 163:105910. https://doi.org/10.1016/j.microc.2020.105910
Anitha V, Khadar AMA, Kalluraya B (2006) Simultaneous determination of titanium and molybdenum in steel samples using derivative spectrophotometry in neutral micellar medium. Spectrochim Acta A Mol Biomol Spectrosc 64:383–390. https://doi.org/10.1016/j.saa.2005.07.034
Kezia S, AnithaV LG (2017) Flavonol based surface modification of doped chalcogenide nanoflakes as an ultrasensitive fluorescence probe for Al3+ ion. Anal Chim Acta 992:94–104. https://doi.org/10.1016/j.aca.2017.08.045
Ajay Piriya VS, Printo Joseph, Kiruba Daniel SCG, Susithra Lakshmanan, Takatoshi Kinoshita, Muthusamy Sivakumar (2017) Colorimetric sensors for rapid detection of various analytes.Materials mater.sci.Eng.A 78:1231–1245. https://doi.org/10.1016/j.msec.2017.05.018
Sarkar D, Pramanik AK, Mondal TK (2014) Coumarin based ‘turn-on’ fluorescent chemosensor for Zn2+ and and HSO4−: an experimental and theoretical study. RSC Adv 4:25341–25347. https://doi.org/10.1039/C4RA02765E
Egorova OA, Seo H, Amrita C, Ann KH (2010) Reaction-based fluorescent sensing of Au(I)/Au(III) species: mechanistic implications on vinyl gold intermediates. Org Lett 12:401–403. https://doi.org/10.1021/ol902395x
Boens N, Leen V, Dehaen W (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41:1130–1172. https://doi.org/10.1039/C1CS15132K
Akshaya KB, Varghese A, Lobo PL, Kumari R, George L (2016) Synthesis and photophysical properties of a novel phthalimide derivative using solvatochromic shift method for the estimation of ground and singlet excited state dipole moments. J Mol Liq 224:247–254. https://doi.org/10.1016/j.molliq.2016.09.115
Pearce DA, Jotterand N, Carrico IS, Imperiali B (2001) Derivatives of 8-Hydroxy-2-methylquinoline Are Powerful Prototypes for Zinc Sensors in Biological Systems. J Am Chem Soc 123:5160–5161. https://doi.org/10.1021/ja0039839
Sousa M, Pinto M (2005) Synthesis of Xanthones: An Overview. Curr Med Chem 12:2447–2479. https://doi.org/10.2174/092986705774370736
Grim JB, Lavis LD (2011) Synthesis of rhodamine from fluoresceins using Pd catalysed C-N Cross Coupling. Org Lett 13:6354. https://doi.org/10.1021/ol202618t
Adamczyk M, Grote J (2001) Efficient fluorescein spirolactam and bisspirolactam synthesis. Synth Commun 31:2681–2690. https://doi.org/10.1081/SCC-100105396
Oliveira E, Bértolo E, Núñez C, Pilla V, Santos HM, Fernández-Lodeiro J, Fernández-Lodeiro A, Djafari J, Capelo JL, Lodeiro C (2017) Green and Red fluorescent dyes for translational applications in imaging and sensing analytes: A dual-color flag. ChemistryOpen 7:9–52. https://doi.org/10.1002/open.201700135
Rajasekar M (2020) Recent Development in Fluorescein derivatives. J Mol Struct 1224:129085. https://doi.org/10.1016/j.molstruc.2020.129085
Banks WA, Kastin AJ, Durham DA (1989) Bidirectional transport of interleukin-1 alpha across the blood-brain barrier. Brain Res Bull 23:433–437. https://doi.org/10.1016/0361-9230(89)90185-8
Zheng H, Zhan XQ, Bian QN, Zhang XJ (2013) Advances in modifying fluorescein and rhodamine fluorophores as fluorescent chemosensors. Chem Commun 49:429–447. https://doi.org/10.1039/C2CC35997A
Shabir G, Saeed A, Channar PA (2017) A Review on the Recent Trends in Synthetic Strategies and Applications of Xanthene Dyes. Mini Rev Org Chem 15:166–197. https://doi.org/10.2174/1570193X14666170518130008
Yan F, Fan K, Zhang R, Zu F, Xu J, Li X (2017) Fluorescein applications as fluorescent probes for the detection of analytes. TrAC Trends Anal Chem 97:15–35. https://doi.org/10.1016/j.trac.2017.08.013
Bayraktutan T, Onganer Y, Meral K (2016) Polyelectrolyte-induced H-aggregation of Merocyanine 540 and its application in metal ions detection as a colorimetric sensor. Sens Actuators B Chem 226:52–61. https://doi.org/10.1016/j.snb.2015.11.115
Kaur M, Cho MJ, Choi DH (2016) A phenothiazine-based naked-eye fluorescent probe for the dual detection of Hg2+ and Cu2+: application as a solid state sensor. Dyes Pigm 125:1–7. https://doi.org/10.1016/j.dyepig.2015.09.030
Miyashita Y, Mori J, Yokoyama H, Yoshikane M, Watanabe H, Takeuchi K et al (2008) A new model system for studying excited states of dye aggregates of photographic color paper. J Photochem Photobiol A: Chem 194:129–135. https://doi.org/10.1016/j.jphotochem.2007.07.025
Walker BJ, Dorn A, Bulovic V, Bawendi MG (2011) Color-selective photocurrent enhancement in coupled J-aggregate/nanowires formed in solution. Nano Lett 11:2655–2659. https://doi.org/10.1021/nl200679n
Egawa T, Koide Y, Hanaoka K, Komatsu T, Terai T, Nagano T (2011) Development of a fluorescein analogue, TokyoMagenta, as a novel scaffold for fluorescence probes in red region. Chem Commun 47:4162–4164. https://doi.org/10.1039/C1CC00078K
Czarnik AW (1993) Fluorescent Chemosensors for Ion and Molecular Recognition. American Chemical Society, Washington DC, USA
Juan Z, Swager TM (2005) Poly (arylene ethynylene) s in chemosensing and biosensing. Adv Polym Sci 177:151–179. https://doi.org/10.1007/b101377
Thomas W, Joly GD, Swager TM (2007) Chemical sensors based on amplifying fluorescent conjugated polymers. Chem Rev 107:1339–1386. https://doi.org/10.1021/cr0501339
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40. https://doi.org/10.1016/S0010-8545(00)00246-0
Gunnlaugsson T, Glynn M, Tocci GM, Kruger PE, Pfeffer FM (2006) Anion recognition and sensing in organic and aqueous media using luminescent and colorimetric sensors. Coord Chem Rev 250:3094. https://doi.org/10.1016/j.ccr.2006.08.017
Sjöback R, Nygren J, Kubista M (1995) Absorption and fluorescence properties of fluorescein. Spectrochim Acta A Mol Biomol Spectrosc 51:L7–L21. https://doi.org/10.1016/0584-8539(95)01421-P
Aysha TS, El-Sedik MS, Mohamed MBI, Gaballah ST, Kamel MM (2019) Dual functional colorimetric and turn-off fluorescence probe based on pyrrolinone ester hydrazone dye derivative for Cu2+ monitoring and pH change. Dyes Pigm 170:107549. https://doi.org/10.1016/j.dyepig.2019.107549
Kurita M, Momma M, Mizuguchi K, Nakano H (2013) Fluorescence Color Change of Aggregation-Induced Emission of 4-[Bis(4-methylphenyl)amino]benzaldehyde. Chem Phys Chem 14:3898–3901. https://doi.org/10.1002/cphc.201300781
Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J (2008) A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem Soc Rev 37:1465–1472. https://doi.org/10.1039/B802497A
Quang DT, Kim JS (2010) Fluoro- and Chromogenic Chemodosimeters for Heavy Metal Ion Detection in Solution and Biospecimens. Chem Rev 110:6280–6301. https://doi.org/10.1021/cr100154p
Terai T, Nagano T (2008) Fluorescent probes for bioimaging applications. Curr Opin Chem Biol 12:515–521. https://doi.org/10.1016/j.cbpa.2008.08.007
Valeur B, Leray I (2003) A highly sensitive and selective fluorescent molecular sensor for Pb (II) based on a calix [4] arene bearing four dansyl groups. Chem Commun 8:996–997. https://doi.org/10.1039/B301323E
Shamsipur M, Alizadeh K, Hosseini M, Caltagiroe C, Lippolis V (2006) A selective optode membrane for silver ion based on fluorescence quenching of the dansylamidopropyl pendant arm derivative of 1-aza-4, 7, 10-trithiacyclododecane. Sens Actuators B Chem 113:892–899. https://doi.org/10.1016/j.snb.2005.03.117
Aragoni MC, Arca M, Bencini A, Blake AJ, Caltagirone C, Filippo GD, Devillanova FA, Garau A, Gelbrich T, Hurssthouse MB et al (2007) Tuning the Selectivity/Specificity of Fluorescent Metal Ion Sensors Based on N2S2 Pyridine-Containing Macrocyclic Ligands by Changing the Fluorogenic Subunit. Inorg Chem 46:4548–4559. https://doi.org/10.1021/ic070169e
Thorfinnur G, Haslin DPA, Mark G, Paul EK, Gillian MH, Frederick MP, Gdos CM, S, Juliann T (2005) Fluorescent Photoinduced Electron Transfer (PET) Sensors for Anions; From Design to Potential Application. J Fluoresc 15:287–299. https://doi.org/10.1007/s10895-005-2627-y
Hao F, Gurpreet K, Binghe W (2004) Progress in Boronic Acid-Based Fluorescent Glucose Sensors. J Fluoresc 14:–489. https://doi.org/10.1023/B:JOFL.0000039336.51399.3b
Pydisetti GR, Birudaraju S, Siva TR (2019) Colorimetric and turn-on Fluorescence Chemosensor for Hg2+ Ion Detection in Aqueous Media. J Fluoresc 29:353–360. https://doi.org/10.1007/s10895-018-02342-4
Kumar M, Kumar N, Bhalla V, Sharma PR, Kaur T (2011) Highly selective Fluorescence Turn-on Chemodosimeter Based on Rhodamine for Nanomolar Detection of Copper Ions. Org Lett 14:406–409. https://doi.org/10.1021/ol203186b
Strausak D, Mercer JFB, Dieter HH, Stremmel W, Multhaup G (2001) Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson disease. Brain Res Bull 55:175–185. https://doi.org/10.1016/s0361-9230(01)00454-3
Stelmashook EV, Isaev NK, Genrikhs EE, Amelkina GA, Khaspekov LG, Skrebitsky VG, Illarioshkin SN (2014) Role of zinc and copper ions in the pathogenetic mechanisms of Alzheimer’s and Parkinson’s diseases. Biochemistry 79:391–396. https://doi.org/10.1134/S0006297914050022
Cakic M, Mitic Z, Nikolic SI, Savic IM (2013) Design and optimization of drugs used to treat copper deficiency. Expert Opin Drug Discov 8:1253–1263. https://doi.org/10.1517/17460441.2013.825245
Sivaraman G, Iniya M , Kotla N.G, Singaravadivel S, Gulyani A, Chellappa D (2018) Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord Chem Rev 357 50–104. https://doi.org/10.1016/j.ccr.2017.11.020
Diwana U, Kumar A, Kumar V, Upadhyaya KK, Roychowdhury PK (2014) A water compatible turn ‘on’ optical probe for Cu2+ based on a fluorescein–sugar conjugate. Sens Actuators B Chem 196:345–435. https://doi.org/10.1016/j.snb.2014.02.031
Jesionowski T, Nowacka M, Ciesielczyk F (2012) Electrokinetic properties of hybrid pigments obtained via adsorption of organic dyes on the silica support. Pigment Resin Technol 41:9–19. https://doi.org/10.1108/03699421211192235
Jesionowski T, Przybylska A, Kurc B, Ciesielczyk F (2011) Hybrid pigments preparation via adsorption of CI Mordant Red 3 on both unmodified and aminosilane–functionalised silica supports. Dyes Pigm 89:127–136. https://doi.org/10.1016/j.dyepig.2010.09.014
Chen X, Tong A (2012) Modification of silica nanoparticles with fluorescein hydrozide for Cu(II)sensing. Dyes Pigm 95:776–783. https://doi.org/10.1016/j.dyepig.2012.06.012
Bao X, Cao Q, Wu X, Shu H, Zhou B, Geng Y, Zhu J (2016) Design and synthesis of a new selective fluorescent chemical sensor for Cu2+ based on a Pyrrole moiety and a Fluorescein conjugate. Tetrahedron Lett 57:942–948. https://doi.org/10.1016/j.tetlet.2016.01.056
Li T, Yang Z, Li Y, Liu Z, Qi G, Wang B (2011) A novel fluorescein derivative as a colorimetric chemosensor for detecting copper(II) ion. Dyes Pigm 88:103–108. https://doi.org/10.1016/j.dyepig.2010.05.008
Elmorsi TM, Aysha TS, Machalicky O, Mohamed MBI, Bedair AH (2017) A dual functional colorimetric and fluorescence chemosensor based on benzo[f]fluorescein dye derivatives for copper ions and pH; kinetics and thermodynamic study. Sens Actuators B Chem 253:437–450. https://doi.org/10.1016/j.snb.2017.06.084
Rathod R, Bera S, Singh M, Mondal D (2016) A Colorimetric and Fluorometric Investigation of Cu(II) ion in Aqueous Medium with a Fluorescein-based Chemosensor. RSC Adv 6:34608–34615. https://doi.org/10.1039/C6RA03021A
Wua X, Donga GX, W, Maa J, Chaoa J, Lic C, Wanga Li, Dong C (2016) A novel fluorescein-based colorimetric probe for Cu2+ detection. RSC Adv 6:59677–59683. https://doi.org/10.1039/C6RA07236D
Zhang J, Li Z, Wei Y, Ma J, Shuang S, Cai Z, Dong C (2014) A selectively fluorescein-based colorimetric probe for detecting copper(II) ion. Spectrochim Acta A Mol Biomol Spectrosc 122:731–736. https://doi.org/10.1016/j.saa.2013.11.096
Chen X, Ma H (2006) A selective fluorescence-on reaction of spiro form fluorescein hydrazide with Cu(II). Anal Chim Acta 575:217–222. https://doi.org/10.1016/j.aca.2006.05.097
Liu G, Ren P, Yang F, Dou X, Wang J, Song Y (2018) Two novel colorimetric probes (5-HMBA-FH and 3-HMBA-FH) based on fluorescein for copper(II) ion detection. Can J Chem 96:1037–1045. https://doi.org/10.1139/cjc-2018-0105
Volkhard Helms (2008) Fluorescence Resonance Energy Transfer. Principles of Computational Cell Biology, 202, Wiley-VCH: Weinheim.
Mondal S, Manna SK, Maiti K, Maji R, Ali SS, Manna S, Mandal S, Uddin MdR, Mahapatra AK (2017) Phenanthroline-fluorescein molecular hybrid as a ratiometric and selective fluorescent chemosensor for Cu2+ via FRET strategy: synthesis, computational studies and in vitro applications. Supramol Chem 29:616–626. https://doi.org/10.1080/10610278.2017.1301452
Wanga S, Wanga X, Zhanga Z, Chen L (2015) Highly sensitive fluorescence detection of copper ion based on its catalytic oxidation to cysteine indicated by fluorescein isothiocyanate functionalized gold nanoparticles. Colloids Surf A Physicochem Eng Asp 468:333–338. https://doi.org/10.1016/j.colsurfa.2014.12.050
Mahajan PG, Dige NC, Vanjare B, Hui Eo S, Kim SJ, Hwan LK (2019) A nano sensor for sensitive and selective detection of Cu2+ based on fluorescein: Cell imaging and drinking water analysis. Spectrochim Acta A Mol Biomol Spectrosc 216:105–116. https://doi.org/10.1016/j.saa.2019.03.021
Helal A, Kim HS, Zain H, Yamani NS, M (2015) Fluorescein-N-Methylimidazole Conjugate as Cu2+ Sensor in Mixed Aqueous Media Through Electron Transfer. J Fluoresc 26:1–9. https://doi.org/10.1007/s10895-015-1713-z
Ma L, Liu G, Pu S, Ding H, Li G (2016) A highly selective fluorescent chemosensor for Cu2+ based on a new diarylethene with triazole-linked fluorescein. Tetrahedron 72:985–991. https://doi.org/10.1016/J.TET.2015.12.068
Fan C, Luo S, Liu R (2015) Optimization of an analytical method for the spectrophotometric determination of copper in tea and water samples after ultrasonic assisted cloud point extraction using a benzothiazole fluorescein derivative complexing agent. RSC Adv 5:65321–65327. https://doi.org/10.1039/C5RA11212E
Lambert KF, Evers DC, Warner KA, King SL, Selin NE (2012) Integrating mercury science and policy in the marine context: Challenges and opportunities. Environ Res 119:132–142. https://doi.org/10.1016/j.envres.2012.06.002
Syversen T, Kaur P (2012) The toxicology of mercury and its compounds. J Trace Elem Med Biol 26:215–226. https://doi.org/10.1016/j.jtemb.2012.02.004
Ma YH, Zhang Z, Xu YL, Ma M, Chen B, Wei L, Xiao LH (2016) A bright carbon-dot based fluorescent probe for selective and sensitive detection of mercury ions. Talanta 161:476–481. https://doi.org/10.1016/j.talanta.2016.08.082
Kb A, G R, Sasitharan K, TP V, Varghese A, George L (2019) Trace level determination of Hg2+ ions in environmental samples with mercaptotriazole-functionalized TiO2 nanostructure-based fluorescent probe. Anal Methods 11:537–547. https://doi.org/10.1039/C8AY02109K
Sparano BA, Shahi SP, Koide K (2004) Effect of binding and conformation on fluorescence quenching in new 2′,7′-dichlorofluorescein derivatives. Org Lett 6:1947–1949. https://doi.org/10.1021/ol049537y
Kim HJ, Park JE, Choi MG, Ahn S, Chang S (2010) Selective chromogenic and fluorogenic signalling of Hg2+ ions using a fluorescein-coumarin conjugate. Dyes Pigm 84:54–58. https://doi.org/10.1016/j.dyepig.2009.06.009
Taki M, Iyoshi S, Ojida A, Hamachi I, Yamamoto YJ (2010) Development of highly sensitive fluorescent probes for detection of intracellular copper (I) in living systems. Am Chem Soc 132:5938–5939. https://doi.org/10.1021/ja100714p
Ruan YB, Xie J (2011) Unexpected highly selective fluorescence ‘turn-on’ and ratiometric detection of Hg2+ based on fluorescein platform. Tetrahedron 67:8717–8723. https://doi.org/10.1016/j.tet.2011.09.028
Feng Y, Kuai Z, Song Y, Guo J, Yang Q, Shan Y, Li Y (2017) A novel “turn-on” thiooxofluorescein-based colorimetric and fluorescent sensor for Hg2+ and its application in living cells. Talanta 170:103–110. https://doi.org/10.1016/j.talanta.2017.03.099
Guang S, Tian J, Wei G, Yan Z, Pan H, Feng J, Xu H (2017) A modified fluorescein derivative with improved water-solubility for turn-on fluorescent determination of Hg2+ in aqueous and living cells. Talanta 170:89–96. https://doi.org/10.1016/j.talanta.2017.03.108
Liu D, Wang Y, Wang R, Wang B, Chang H, Chen J, Yang G, He H (2018) Fluorescein-based fluorescent sensor with high selectivity for mercury and its imaging in living cells. Inorg Chem Commun 89:46–50. https://doi.org/10.1016/j.inoche.2018.01.016
Qu Z, Wang L, Fang S, Qin D, Zhou J, Yong G, Duan H (2019) Fluorescein-immobilized optical hydrogels: Synthesis and its application for detection of Hg2+. Microchem J 150:104198. https://www.x-mol.com/paperRedirect/5820391
Bothwell TH, Charlton RW, Cook JD, Finch CA (1979) Iron Metabolism in Man. Blackwell Scientific, Oxford
Crichton, RR (2001) Inorganic Biochemistry of Iron Metabolism; John Wiley & Sons: West Sussex
Ma Y, Hider RC (2009) The selective quantification of iron by hexadentate fluorescent probes. Bioorg Med Chem 17:8093–8101. https://doi.org/10.1016/j.bmc.2009.09.052
Gao Y, Liu H, Liu Q, Wang W (2016) A novel colorimetric and OFF-ON fluorescent chemosensor based on fluorescein derivative for the detection of Fe3+ in aqueous solution and living cells. Tetrahedron Lett 17:1852–1855. https://doi.org/10.1016/j.tetlet.2016.03.050
Queirós C, Silva AMG, Lopes SC, Ivanova G, Gameiro P, Rangel M (2012) A novel fluorescein-based dye containing a catechol chelating unit to sense iron(III). Dyes Pigm 93:1447–1455. https://doi.org/10.1016/j.dyepig.2011.10.010
Ma T, Zhao X, Matsuo Y, Song J, Zhao R, Faheem M, Chen M, Zhang Y, Zhu TY, G, (2019) Fluorescein-based fluorescent porous aromatic framework for Fe3+ detection with high sensitivity. J Mater Chem C 7:2327–2332. https://doi.org/10.1039/C8TC06288A
Ays Merve S, Onganer Y, Meral K (2017) An unusual “off-on” fluorescence sensor for iron(III) detection based on fluorescein–reduced graphene oxide functionalized with polyethyleneimine. Sens Actuators B Chem 239:343–351. https://doi.org/10.1016/j.snb.2016.08.025
Kumar A, Rao GK, Saleem F, Kumar.R, Singh A.K Hazard J (2014) Palladium (ii) complexes bearing the 1, 2, 3-triazole based organosulfur/selenium ligand: synthesis, structure and applications in Heck and Suzuki-Miyaura coupling. RSC Adv 4:56102–56111. https://doi.org/10.1039/C4RA09574J
Reddy G U, Ali F, Taye N, Chattopadhyay S, Das A. (2015) A new turn on Pd2+ -specific fluorescence probe and its use as an Imaging reagent for cellular uptake in Hct116 cells. Chem Commun 51: 3649–3652https://doi.org/10.1039/C4CC10171E
Wiseman CLS, Zereini F (2009) A review of recent evidence Total Environ. Sci. Airborne particulate matter, platinum group elements and human health. Sci Total Environ 407:2493–2500. https://doi.org/10.1016/j.scitotenv.2008.12.057
Spicer CD, Triemer T, Davis BG, Am J (2014) Palladium-Mediated Cell-Surface Labeling. J Am Chem Soc 134:800–803. https://doi.org/10.1021/ja209352s
Yusop RM, Unciti-Broceta A, Johansson EMV, Sánchez-Martín RM, Bradley M (2011) Palladium-mediated intracellular chemistry. Nat Chem 3:239–243. https://doi.org/10.1038/nchem.981
Santra M, Kyun Ko S, Shin I, Han Ahn K (2010) Fluorescent detection of palladium species with an O-propargylated fluorescein. Chem Commun 46:3964–3966. https://doi.org/10.1039/C001922D
Kitley WR, Santa Maria PJ, Cloyd RA, Laura MW (2012) Synthesis of High Contrast Fluorescein-Diethers for Rapid Bench-Top Sensing of Palladium. Chem Commun 51:8520–8523. https://doi.org/10.1039/C5CC02192H
Panchompoo J, Aldous L, Baker M, Wallace MI, Richard GC (2012) One-step synthesis of fluorescein modified nano-carbon for Pd(II) detection via fluorescence quenching. Analyst 137:2054–2062. https://doi.org/10.1039/C2AN16261J
Wei G, Wang L, Jiao J, Hou J, Cheng Y, Zhu C (2012) Cu2+ triggered fluorescence sensor based on fluorescein derivative for Pd2+ detection. Tetrahedron Lett 53:3459–3462. https://doi.org/10.1016/j.tetlet.2012.04.108
Silva JD, Williams R (2001) The biological chemistry of the elements. Oxford University Press
Beyersmann D, Haase H (2001) Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 14:331–341. https://doi.org/10.1023/A:1012905406548
Nriagu J (2011) Zinc toxicity in humans. Encycl Environ Heal pp. 801–807
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462. https://doi.org/10.1038/nrn1671
Wang D, Xiang X, Yang X, Wang X, Guo Y, Liu W, Qin W (2014) Fluorescein-based chromo-fluorescent probe for zinc in aqueous solution: spirolactam ring opened or closed? Sens Actuators B Chem 201:246–254. https://doi.org/10.1016/j.snb.2014.05.019
An J, Yan M, Yang Z, Li T, Zhou Q (2013)A turn-on fluorescent sensor for Zn (II) based on fluorescein-coumarin conjugate. Dyes Pigm 99:1–5. https://doi.org/10.1016/j.dyepig.2013.04.018
Chantalakana K, Choengchan N, Yingyuad P, Thongyoo P (2016) A highly selective ‘turn-on’ fluorescent sensor for Zn2+ based on fluorescein conjugates. Tetrahedron Lett 57:1146–1149. https://doi.org/10.1016/j.tetlet.2016.01.106
Clark MA, Duffy K, Tibrewala J, Lippard SJ (2003) Synthesis and metal-binding properties of chelating fluorescein derivatives. Org Lett 5:2051–2054. https://doi.org/10.1021/ol0344570
Vidya B, Sivaraman G, Sumesh RS, Chellappa D (2016) Fluorescein-Based ‘“Turn On”’ Fluorescence Detection of Zn2+ and Its Applications in Imaging of Zn2+ in Apoptotic Cells. ChemistrySelect 1:4024–4029. https://doi.org/10.1002/slct.201600863
Kumari R, George VAL (2017) Estimation of Ground-State and Singlet Excited-State Dipole Moments of Substituted Schiff Bases Containing Oxazolidin-2-one Moiety through Solvatochromic Methods. J Fluoresc 27:151–165. https://doi.org/10.1007/s10895-016-1942-9
Das B, Jana A, Mahapatra AD, Chattopadhyay D, Dhara A, Mabhai S, Dey S (2019) Fluorescein derived Schiff base as fluorimetric zinc (II) sensor via ‘turn on’ response and its application in live cell imaging. Spectrochim Acta A Mol Biomol Spectrosc 212:222–231. https://doi.org/10.1016/j.saa.2018.12.053
Erdemir S, Tabakci B (2017) Selective and Sensitive Fluorescein-Benzothiazole Based Fluorescent Sensor for Zn2+ Ion in Aqueous Media. J Fluoresc 27:2145–2152. https://doi.org/10.1007/s10895-017-2153-8
Satarug S, Garrett SH, Sens MA, Sens DA (2010) Cadmium, Environmental Exposure, and Health Outcomes. Environ Health Perspect 118:182–190. https://doi.org/10.1289/ehp.0901234
Sabir S, Akash MSH, Fiayyaz F, Saleem U, Mehmood MH, Rehman K (2019) Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomed Pharmacother 114:108802. https://doi.org/10.1016/j.biopha.2019.108802
Sotomayor CG, Groothof D, Eisenga VJ, Knobbe JMF et al (2021) Plasma cadmium is associated with increased risk of long-term kidney graft failure. Kidney Int 99:1213–1224. https://doi.org/10.1016/j.kint.2020.08.027
Bernhoft RA (2013) Cadmium Toxicity and Treatment. Sci World J 2013:1–7. https://doi.org/10.1155/2013/394652
Rana MN, Tangpong J, Rahman MM (2018) Toxicodynamics of Lead, Cadmium, Mercury and Arsenic- induced kidney toxicity and treatment strategy: A mini review. Toxicol Rep 5:704–713. https://doi.org/10.1016/j.toxrep.2018.05.012
Pak Y, Swamy K, Yoon J (2015) Recent Progress in Fluorescent Imaging Probes. Sensors 15:24374–24396. https://doi.org/10.3390/s150924374
Gui R, An X, Huang W (2013) An improved method for ratiometric fluorescence detection of pH and Cd2+ using fluorescein isothiocyanate–quantum dots conjugates. Anal Chim Acta 767:134–140. https://doi.org/10.1016/j.aca.2013.01.006
Liu X, Liu D, Qi J, Cui Z, Chang H, He H, Yang G (2015) A new fluorescent sensor for Cd2+ and its application in living cells imaging. Tetrahedron Lett 56:1322–1327. https://doi.org/10.1016/j.tetlet.2015.02.010
Irie M, Fukaminato T, Matsuda K, Kobatake S (2014) Photochromism of Diarylethene Molecules and Crystals: Memories, Switches, and Actuators. Chem Rev 114:12174–12277. https://doi.org/10.1021/cr500249p
Li G, Liu G, Zhang D, Pu S (2016) A new fluorescence probe based on fluorescein-diarylethene fluorescence resonance energy transfer system for rapid detection of Cd2+. Tetrahedron 72:6390–6396. https://doi.org/10.1016/j.tet.2016.08.037
Janning C, Willbold E, Vogt C, Nellesen J, Meyer-Lindenberg A, Windhagen H, Thorey F, Witte F (2010) Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater 6:1861–1868. https://doi.org/10.1016/j.actbio.2009.12.037
Martinez-Jimenez MI, Garcia-Gomez S, Bebenek K, Sastre-Moreno G, Calvo PA, Diaz-Talavera A, Kunkel TA, Blanco L (2015) Alternative solutions and new scenarios for translesion DNA synthesis by human PrimPol. DNA Repair 29:127–138. https://doi.org/10.1016/j.dnarep.2015.02.013
Rude RK, Gruber HE (2004) Magnesium deficiency and osteoporosis: animal and human observations. J Nutr Biochem 15:710–716. https://doi.org/10.1016/j.jnutbio.2004.08.001
Svagzdiene M, Sirvinskas E, Baranauskiene D, Adukauskiene D (2015) Correlation of magnesium deficiency with C-reactive protein in elective cardiac surgery with cardiopulmonary bypass for ischemic heart disease. Medicina 51:100–106. https://doi.org/10.1016/j.medici.2015.03.003
Moncayo R, Moncayo H (2014) Exploring the aspect of psychosomatics in hypothyroidism: The WOMED model of body-mind interactions based on musculoskeletal changes psychological stressors, and low levels of magnesium. Woman-Psychosom Gynaecol Obst 1:1–11. https://doi.org/10.1016/j.woman.2014.02.001
Ge F, Yang C, Cai Z (2015) Fluorescence Sensor Performance of a New Fluorescein Derivate: [2-Morpholine-4-(6-chlorine-1,3,5-s-triazine)-amino]fluorescein. Bull Korean Chem Soc 36:2703–2709. https://doi.org/10.1002/bkcs.10551
Cho H, Hee Seo S, Na Y, Kwon Y (2018) The Synthesis and Anticancer Activities of Chiral Epoxy-substituted Chromone Analogs. Bioorg Chem 84:347–354. https://doi.org/10.1016/j.bioorg.2018.11.054
Li C, Li S, Wang G, Yang Z (2015) Spectroscopic properties of a chromone-fluorescein conjugate as Mg2+ “turn on” fluorescent probe. J Photoch Photobio A 356:700–707. https://doi.org/10.1016/j.jphotochem.2016.03.001
Hou L, Feng J, Wang Y, Dong C, Shuang S, Wang Y (2017) Single fluorescein-based probe for selective colorimetric and fluorometric dual sensing of Al3+ and Cu2+. Sens Actuators B 247:451–460. https://doi.org/10.1016/j.snb.2017.03.027
Zhao G, Wei G, Yan Z, Guo B, Guang S, Wu R, Xu H (2019) A multiple fluorescein-based turn-on fluorophore (FHCS) identified for simultaneous determination and living imaging of toxic Al3+ and Zn2+ by improved Stokes shift. Anal Chim Acta 1095:185–196. https://doi.org/10.1016/j.aca.2019.10.025
Kaura P, Lala B, Kaura N, Singhb G, Singhb A, Kaurc G, Singh J (2019) Selective two way Cd(II) and Co(II) ions detection by 1,2,3–triazole linked fluorescein derivative. J Photoch Photobio A 382:111847. https://doi.org/10.1016/j.jphotochem.2019.05.010
Erdemir S, Kocyigit O (2017) A novel dye based on phenolphthalein-fluorescein as a fluorescent probe for the dual-channel detection of Hg2+ and Zn2+. Dyes Pigm 145:72–79. https://doi.org/10.1016/j.dyepig.2017.05.053
Gale PA, Caltagirone C (2017) Fluorescent and colorimetric sensors for anionic species. Coord Chem Rev 354:2–27. https://doi.org/10.1016/j.ccr.2017.05.003
Kaur N, Kaur G, Fegade UA, Singh A, Sahoo SK, Kuwar AS, Singh N (2017) Anion sensing with chemosensors having multiple NH recognition units. Trac-Trend Anal Chem 95:86–109. https://doi.org/10.1016/j.trac.2017.08.003
Anjaneyulu L, Kumar EA, Sankannavar R, Rao KK (2012) Defluoridation of Drinking Water and Rainwater Harvesting Using a Solar Still. Ind Eng Chem Res 51:8040–8048. https://doi.org/10.1021/ie201692q
Liu JM, Lin LP, Wang XX, Jiao L, Cui ML, Jiang SL, Cai WL, Zhang LH, Zeng ZY (2013) Zr(H2O)2EDTA modulated luminescent carbon dots as fluorescent probes for fluoride detection. Analyst 138:278–283. https://doi.org/10.1039/C2AN36055A
U.S. Department of Health and Human Services Federal Panel on Community Water Fluoridation (2015) U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public Health Reports 130:318–331. https://doi.org/10.1177/003335491513000408
Renuga D, Udhayakumari D, Suganya S, Velmathi S (2012) Novel thiophene based colorimetric and fluorescent receptor for selective recognition of fluoride ions. Tetrahedron Lett 53:5068–5070. https://doi.org/10.1016/j.tetlet.2012.06.147
Song P, Ding JX, Chu TS (2012) TD-DFT study on the excited-state proton transfer in the fluoride sensing of a turn-off type fluorescent chemosensor based on anthracene derivatives. Spectrochim Acta A Mol Biomol Spectrosc 97:746–752. https://doi.org/10.1016/j.saa.2012.07.010
Zimmerman JR, Criss C, Evans S, Ernst M, Nieszala M, Stafford A, Szczerba J (2018) Fluorescent sensor for fluoride anion based on a sulfonamido-chromone scaffold. Tetrahedron Lett 59:2473–2476. https://doi.org/10.1016/j.tetlet.2018.05.050
Yang X-F, Wang L, Xu H, Zhao, (2009) A fluorescein-based fluorogenic and chromogenic chemodosimeter for the sensitive detection of sulfide anion in aqueous solution. M Anal Chim Acta 631:91–95. https://doi.org/10.1016/j.aca.2008.10.037
Asthana SK, Kumar A, Neeraj, Upadhyay KK (2014) A reaction based chromofluorogenic turn-on probe for specific detection of fluoride over sulfide/thiols. Tetrahedron Lett 55:5988–5992. https://www.x-mol.com/paperRedirect/4011186
Ashokkumar P, Weiboff H, Kraus W, Rurack K (2014) Test-stripbased fluorometric detection of fluoride in aqueous media with a BODIPY-linked hydrogen-bonding receptor. Angew Chem Int Ed 53:2225–2229. https://doi.org/10.1002/anie.201307848
Zhang P, Li C, Zhang H, Li Y, Yu X, Geng L et al (2015) Fluorogenic and chromogenic detection of biologically important fluoride anion in aqueous media with a fluorescein-linked hydrogen-bonding receptor via “off–on” approach. J Incl Phenom Macrocycl Chem 81:295–300. https://doi.org/10.1007/s10847-014-0456-9
Kim HY, Im HG, Chang S (2015) Colorimetric and fluorogenic signaling of fluoride ions by thiophosphinated dichlorofluorescein. Dyes Pigm 112:170–175. https://doi.org/10.1016/j.dyepig.2014.06.030
Jiao S, Wang X, Sun Y, Zhang L, Sun W, Sun Y, Wang X, Ma P, Song D (2018) A novel fluorescein-coumarin-based fluorescent probe for fluoride ions and its applications in imaging of living cells and zebrafish in vivo. Sens Actuators B 262:188–194. https://doi.org/10.1016/j.snb.2018.01.186
Gungor N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, Godschalk RW, Schooten FJ (2010) Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis 25:149–154. https://doi.org/10.1093/mutage/gep053
Xu Q, Lee K-A, Lee S, Lee KM, Lee W-J, Yoon J (2013) A Highly Specific Fluorescent Probe for Hypochlorous Acid and Its Application in Imaging Microbe-Induced HOCl Production. J Am Chem Soc 135:9944–9949. https://doi.org/10.1021/ja404649m
Samanta S, Halder S, Manna U, Das G (2019) Specific detection of hypochlorite: a cyanine based turn-on fluorescent sensor. J Chem Sci 131:36. https://doi.org/10.1007/s12039-019-1612-y
Taheri M, Mansour N (2019) Functionalized Silicon Nanoparticles as Fluorescent Probe for Detection of Hypochlorite in Water. J Photochem Photobiol A 382:111906. https://doi.org/10.1016/j.jphotochem.2019.111906
Huo FJ, Zhang JJ, Yang YT, Chao JB, Yin CX, Zhang YB, Chen TG (2012) A fluorescein-based highly specific colorimetric and fluorescent probe for hypochlorites in aqueous solution and its application in tap water. Sens Actuators B 166:44–49. https://doi.org/10.1016/j.snb.2011.11.081
Zhou Y, Li J-Y, Chu K-H, Liu K, Yao C, Li J-Y (2012) Fluorescence turn-on detection of hypochlorous acid via HOCl-promoted dihydrofluorescein-ether oxidation and its application in vivo. Chem Commun 48:4677–4679. https://doi.org/10.1039/C2CC30265A
Jin X, Hao L, Hu Y, She M, Shi Y, Obst M, Li J, Shi Z (2013) Two novel fluorescein-based fluorescent probes for hypochlorite and its real applications in tap water and biological imaging. Sens Actuators B 186:56–60. https://doi.org/10.1016/j.snb.2013.05.079
Cheng X, Jia H, Long T, Feng J, Qin J, Li Z (2011) A ‘“turn-on”’ fluorescent probe for hypochlorous acid: convenient synthesis, good sensing performance, and a new design strategy by the removal of CQN isomerization. Chem Commun 47:11978–11980. https://doi.org/10.1039/C1CC15214A
Lie J, Yang X, Zhang D, Liu Y, Tang J, Li Y, Zhao Y, Ye Y A fluorescein-based “turn-on” fluorescence probe for hypochlorous acid detection and its application in cell imaging. Sens.Actuators B,265:85–90, https://doi.org/10.1016/j.snb.2018.03.027
Lv J, Wang F, Wei T, Chen X (2017) Highly Sensitive and Selective Fluorescent Probes for the Detection of HOCl/OCl− Based on Fluorescein Derivatives. Ind Eng Chem Res 56:3757–3764. https://doi.org/10.1021/acs.iecr.7b00381
Wang N, Xu W, Song D, Ma P (2019) A fluorescein-carbazole-based fluorescent probe for imaging of endogenous hypochlorite in living cells and zebrafish. Spectrochim Acta A Mol Biomol Spectrosc 227:117692. https://doi.org/10.1016/j.saa.2019.117692
Jin L, Xu M, Jiang H, Wang W, Wang Q (2018) A Simple Fluorescein Derived Colorimetric and Fluorescent ‘off - on’ Sensor for the Detection of Hypochlorite. Anal Methods 10:4562–4569. https://doi.org/10.1039/C8AY01489B
Prakash M, Shetty MS, Tilak P, Anwar N (2009) Total Thiols: Biomedical Importance and Their Alteration In Various Disorders. Online J Health Allied Sc 8:2,. http://www.ojhas.org/issue30/2009-2-2.htm
Ulrich K, Jakob U (2019) The role of thiols in antioxidant systems. Free Radic Biol Med 140:14–27. https://doi.org/10.1016/j.freeradbiomed.2019.05.035
Cazzola M, Calzetta L, Page C, Rogliani P, Matera MG (2019) Thiol-Based Drugs in Pulmonary Medicine: Much More than Mucolytics. Trends Pharmacol Sci 40:452–463. https://doi.org/10.1016/j.tips.2019.04.015
Marabini L, Rossella C, Pier CB (2011) Protective effect of erdosteine metabolite I against hydrogen peroxide-induced oxidative DNA-damage in lung epithelial cells. Arzneimittelforschung 61:700–706. https://doi.org/10.1055/s-0031-1300590
Baba SP, Bhatnagar A (2018) Role of thiols in oxidative stress. Current Opinion in Toxicol 7:133–139. https://doi.org/10.1016/j.cotox.2018.03.005
Checconi P, Limongi D, Baldelli S, Ciriolo MR, Nencioni L, Palamara AT (2019) Role of Glutathionylation in Infection and Inflammation. Nutrients 11:1952. https://doi.org/10.3390/nu11081952
Barbosa ML, de Meneses A-APM, de Aguiar RPS, de Castro e Sousa JM, de Carvalho Melo Cavalcante AA, Maluf SW, (2020) Oxidative stress, antioxidant defense and depressive disorders: A systematic review of biochemical and molecular markers. J neurol psychiatry brain res 36:65–72. https://doi.org/10.1016/j.npbr.2020.02.006
Atkuri KR, Mantovani JJ, Herzenberg LA (2007) N-Acetylcysteine-a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 7:355–359. https://doi.org/10.1016/j.coph.2007.04.005
Paul BD, Sbodio JI, Xu RS, Vandiver MS, Cha JY, Snowman AM, Snyder SH (2014) Cystathionine gamma-lyase deficiency mediates neurodegeneration in Huntington’s disease. Nature 509:96–100. https://doi.org/10.1038/nature13136
Paul BD, Snyder SH (2014) Neurodegeneration in Huntington’s disease involves loss of cystathionine γ-lyase. Cell Cycle 13:2491–2493. https://doi.org/10.4161/15384101.2014.950538
D P, Saini S, Thakur A, Kumar B, Tyagi S, Nayak MK, (2017) A “Turn-On” thiol functionalized fluorescent carbon quantum dot based chemosensory system for arsenite detection. J Hazard Mater 328:117–126. https://doi.org/10.1016/j.jhazmat.2017.01.015
Sedgwick AC, Gardiner JE, Kim G, Yevglevskis M, Lloyd MD, Jenkins ATA, James TD (2018) Long-wavelength TCF-based fluorescence probes for the detection and intracellular imaging of biological thiols. Chem Commun 54:4786–4789. https://doi.org/10.1039/C8CC01661E
Wang H, Zhou G, Chen X (2013) An iminofluorescein-Cu2+ ensemble probe for selective detection of thiols. Sens Actuators B Chem 176:698–703. https://doi.org/10.1016/j.snb.2012.10.006
Huo F, Kang J, Yin C, Zhang Y, Chao J (2015) A turn-on green fluorescent thiol probe based on the 1,2-addition reaction and its application for bioimaging. Sens Actuators B Chem 207:139–143. https://doi.org/10.1016/j.snb.2014.10.023
Liu Y, Xiang K, Tian B, Zhang J (2016) A fluorescein-based fluorescence probe for the fast detection of thiol. Tetrahedron Lett 57:2478–2483. https://doi.org/10.1016/j.tetlet.2016.04.068
Chen H, Tang Y, Lin W (2016) Recent progress in the fluorescent probes for the specific imaging of small molecular weight thiols in living cells. Trends Analyt Chem 76:166–181. https://doi.org/10.1016/j.trac.2015.11.014
Tian M, Guo F, Sun Y, Zhang W, Miao F, Liu Y et al (2014)A fluorescent probe for intracellular cysteine overcoming the interference by glutathione. Org Biomol Chem 2:6128–6133. https://doi.org/10.1039/C4OB00382A
Manna S, Karmakar P, Ali SS, Guria UN, Sarkar R, Datta P, Mahapatra AK (2018) A Michael addition–cyclization-based switch-on fluorescent chemodosimeter for cysteine and its application in live cell imaging. New J Chem 42:4951–4958. https://doi.org/10.1039/C8NJ00465J
Murale DP, Kim H, Choi WS, Kim Y, Churchill DG (2014) Extremely selective fluorescence detection of cysteine or superoxide with aliphatic ester hydrolysis. RSC Adv 4:46513–46516. https://doi.org/10.1039/C4RA06891B
Liu J, Sun YQ, Zhang H, Huo Y, Shi Y, Shi H et al (2014) A carboxylic acid functionalized coumarinhemicyanine fluorescent dye and its application to construct a fluorescent probe for selective detection of cysteine over homocysteine and glutathione. RSC Adv 4:464542–464550. https://doi.org/10.1039/C4RA10865E
Kand D, Saha T, Talukdar P (2014) Off-on type fluorescent NBD-probe for selective sensing of cysteine and homocysteine over glutathione. Sens Actuators B Chem 196:440–449. https://doi.org/10.1016/j.snb.2014.02.023
Yang X, Guo Y, Strongin RM (2011) Conjugate addition/cyclization sequence enables selective and simultaneous fluorescence detection of cysteine and homocysteine. Angew Chem Int Ed 50:10690–10693. https://doi.org/10.1002/ange.201103759
Wang H, Zhou G, Gai H, Chen X (2012) A fluorescein-based probe with high selectivity to cysteine over homocysteine and glutathione. Chem Commun 48:8341. https://doi.org/10.1039/C2CC33932C
Yang X, Guo Y, Strongin RM (2012) A seminaphthofluorescein-based fluorescent chemodosimeter for the highly selective detection of cysteine. Org Biomol Chem 10:2739–2741. https://doi.org/10.1039/C2OB25178G
Ji Y, Dai F, & Zhou B (2019). Developing a julolidine-fluorescein-based hybrid as a highly sensitive fluorescent probe for sensing and bioimaging cysteine in living cells. Talanta 197:631–637 https://www.x-mol.com/paperRedirect/973897
Staudinger C, Breininger J, Klimant I, Borisov SM (2019) Near-infrared fluorescent aza-BODIPY dyes for sensing and imaging of pH from neutral to highly alkaline range. Analyst 144:2393–2402. https://doi.org/10.1039/C9AN00118B
Escobedo JO, Rusin O, Lim S, Strongin RM (2010) NIR dyes for bioimaging applications. Curr Opin Chem Biol 14:64–70. https://doi.org/10.1016/j.cbpa.2009.10.022
Wang J, Zhou C, Liu W, Zhang J, Zhu X, Liu X, Wang Q, Zhang H (2016) A near-infrared fluorescent probe based on chloroacetate modified naphthofluorescein for selectively detecting cysteine/homocysteine and its application in living cells. Photochem Photobiol Sci 15:1393–1399. https://doi.org/10.1039/C6PP00219F
Chen H, Zhou B, Ye R, Zhu J, Bao X (2017) Synthesis and evaluation of a new fluorescein and rhodamine B-based chemosensor for highly sensitive and selective detection of cysteine over other amino acids and its application in living cell imaging. Sens Actuators B Chem 251:481–489. https://doi.org/10.1016/j.snb.2017.05.078
Fu Z, Han X, Shao Y, Fang J, Zhang Z, Wang Y, Peng Y (2017) Fluorescein-based Chromogenic and Ratiometric Fluorescence Probe for Highly Selective Detection of Cysteine and Its Application in Bioimaging. Anal Chem 89:1937–1944. https://doi.org/10.1021/acs.analchem.6b04431
Scales S, Tsai SP, Zacharias N, Cruz-Chuh J, dela, Bullen G, Velasquez E, … Sadowsky J, (2019) Development of a cysteine-conjugatable disulfide FRET probe: Influence of Charge on Linker Cleavage and Payload Trafficking for an anti-HER2 antibody conjugate. Bioconjugate Chem 30:3046–3056. https://doi.org/10.1021/acs.bioconjchem.9b00678
Hou X, Li Z, Li B, Liu C, Xu Z (2018) An “off-on” fluorescein-based colormetric and fluorescent probe for the detection of glutathione and cysteine over homocysteine and its application for cell imaging. Sens Actuators B Chem 260:295–302. https://doi.org/10.1016/j.snb.2018.01.013
Hou X, Li Z, Wang Y, Li B, Liu C, Zhan Q, Liu G, Wei D, Xu Z (2020) Development of a semiacenaphthenofluorescein-based optical and fluorescent sensor for imaging cysteine in cells. J Photochem Photobiol A Chem 386:112090. https://doi.org/10.1016/j.jphotochem.2019.112090
Heinemann SH, Hoshi T, Westerhausen M, Schiller A (2014) Carbon monoxide – physiology, detection and controlled release. Chem Commun 50(28):3644–3660. https://doi.org/10.1039/C3CC49196J
Romão CC, Blättler WA, Seixas JD, Bernardes GJL (2012) Developing drug molecules for therapy with carbon monoxide. Chem Soc Rev 41:3571. https://doi.org/10.1039/C2CS15317C
Pal S, Mukherjee M, Sen B, Mandal SK, Lohar S, Chattopadhyay P, Dhara K (2015) A new fluorogenic probe for the selective detection of carbon monoxide in aqueous medium based on Pd(0) mediated reaction. Chem Commun 51:4410–4413. https://doi.org/10.1039/C5CC00902B
Zobi F (2013) CO and CO-releasing molecules in medicinal chemistry. Future Med Chem 5:175–188. https://doi.org/10.4155/fmc.12.196
Feng W, Liu D, Feng S, Feng G (2016) Readily Available Fluorescent Probe for Carbon Monoxide Imaging in Living Cells. Anal Chem 88:10648–10653. https://doi.org/10.1021/acs.analchem.6b03073
Feng S, Liu D, Feng W, Feng G (2017) Allyl Fluorescein Ethers as Promising Fluorescent Probes for Carbon Monoxide Imaging in Living Cells. Anal Chem 89:3754–3760. https://doi.org/10.1021/acs.analchem.7b00135
Jimenez M (2010) Hydrogen sulfide as a signaling molecule in the enteric nervous system. Neurogastroenterol Motil 22:1149–1153. https://doi.org/10.1111/j.1365-2982.2010.01600.x
Olas B (2015) Hydrogen sulfide in signaling pathways. Clin Chim Acta 439:212–218. https://doi.org/10.1016/j.cca.2014.10.037
di Masi A, Ascenzi P (2012) H2S: A “Double face” molecule in health and disease. BioFactors 39:186–196. https://doi.org/10.1002/biof.1061
Guidotti TL (2010) Hydrogen Sulfide: advances in understanding human toxicity. Int J Toxicol 29:569–581. https://doi.org/10.1177/1091581810384882
Austigard ÅD, Svendsen K, Heldal KK (2018) Hydrogen sulphide exposure in waste water treatment. J Occup Med Toxicol 13:10. https://doi.org/10.1186/s12995-018-0191-z
Ventura Spagnolo E, Romano G, Zuccarello P, Laudani A, Mondello C, Argo A, Barbera N (2019) Toxicological Investigations in a Fatal and Non-Fatal Accident due to Hydrogen Sulphide (H2S) Poisoning. Forensic Sci Int 300:e4–e8. https://doi.org/10.1016/j.forsciint.2019.04.026
Whiteman M, Winyard PG (2011) Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol 4:13–32. https://doi.org/10.1586/ecp.10.134
Lin VS, Chang CJ (2012) Fluorescent probes for sensing and imaging biological hydrogen sulfide. Curr Opin Chem Biol 16:595–601. https://doi.org/10.1016/j.cbpa.2012.07.014
Kaushik R, Ghosh A, Jose A (2021) Chapter 12 - Colorimetric and fluorescent nanosensors for the detection of gaseous signaling molecule hydrogen sulfide (H2S) Handbook of Nanomaterials for Sensing Applications 203–220. https://doi.org/10.1016/B978-0-12-820783-3.00008-7
Guria UN, Maiti K, Ali SS, Samanta SK, Mandal D, Sarkar R, Mahapatra AK (2018) Reaction-based bi-signaling chemodosimeter probe for selective detection of hydrogen sulfide and cellular studies. New J Chem 42:5367–5375. https://doi.org/10.1039/C7NJ04632D
Hou F, Huang L, Xi P, Cheng J, Zhao X, Xie G, Shi Y, Cheng F, Yia X, Bai D, Zeng Z (2012) A Retrievable and Highly Selective Fluorescent Probe for Monitoring Sulfide and Imaging in Living Cells. Inorg Chem 51:2454–2460. https://doi.org/10.1021/ic2024082
Liu H, Zhao M, Qiao Q, Lang H, Xu J, Xu Z (2014) Fluorescein-derived fluorescent probe for cellular 4 hydrogen sulfide imaging. Chin Chem Lett 25:1060–1064. https://doi.org/10.1016/j.cclet.2014.05.010
Jin X, Wu S, She M, Jia Y, Hao L, Yin B, Wang L, Obst M, Shen Y, ZhangY LJ (2016) A novel fluorescein-based fluorescent probe for detecting H2S and its real applications in blood plasma and biological imaging. Anal Chem 88:11253–11260. https://doi.org/10.1021/acs.analchem.6b04087
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Keerthana S Lieterature data collection; Bincy Sam: Interpretation and writing; Suhakar YN: Critical analysis; Louis George: Reviewing and Editing; Anitha Varghese: Reviewing and Editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
S, K., Sam, B., George, L. et al. Fluorescein Based Fluorescence Sensors for the Selective Sensing of Various Analytes. J Fluoresc 31, 1251–1276 (2021). https://doi.org/10.1007/s10895-021-02770-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02770-9