Abstract
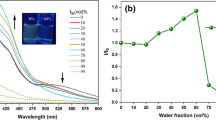
Sila- and germafluorenes containing alkynyl(aryl) substituents at the 2,7- position are strongly emissive with high quantum yields in organic solvents. Provided they are sufficiently soluble in water, their hydrophobic structures have the potential for many biological and industrial applications in the detection and characterization of lipophilic structures. To that end, the emission behaviors of previously synthesized 2,7- bis[alkynyl(biphenyl)]-9,9-diphenylsilafluorene (1), 2,7- bis[alkynyl(methoxynaphthyl)]-9,9-diphenylgermafluorene (2), 2,7- bis[alkynyl(p-tolyl)]-9,9-diphenylsilafluorene (3), and 2,7- bis[alkynyl(m-fluorophenyl)]-9,9-diphenylsilafluorene (4) were characterized in aqueous solution and in the presence of various surfactants. Despite a high degree of hydrophobicity, all of these metallafluorenes (MFs) are soluble in aqueous solution at low micromolar concentrations and luminesce in a common aqueous buffer. Further, the 2,7 substituent makes the emission behavior tunable (up to 30 nm). Fold emission enhancements in the presence of various surfactants are highest toward Triton X-100 and CTAB (ranging from 5 to 25 fold) and are lowest for the anionic surfactants SDS and SDBS. These enhancements are competitive with existing probes of surfactants. Quantum yields in buffer range from 0.11 to 0.34, competitive with many common fluorophores in biological use. Strikingly, MF quantum yields in the presence of TX-100 and CTAB approach 100 % quantum efficiency. MF anisotropies are dramatically increased only in the presence of TX-100, CTAB, and CHAPS. Coupled with the above data, this suggests that MFs associate with neutral and charged surfactant aggregates. Interactions with the anionic surfactants are weaker and/or leave MFs solvent exposed. These properties make metallafluorenes competitive probes for surfactants and their properties and behaviors, and thus could also have important biological applications.









Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CHAPS:
-
3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate
- CMC:
-
critical micelle concentration
- CTAB:
-
cetyltrimethylammonium bromide
- SDS:
-
sodium dodecyl sulfate
- SDBS:
-
Sodium dodecylbenzenesulfonate
- MF:
-
metallafluorene
- TX:
-
Triton X-100
References
Hammerstroem DW, Braddock-Wilking J, Rath NP (2016) Synthesis and characterization of luminescent 2,7-disubstituted silafluorenes. J Organomet Chem 813:110–118
Hammerstroem DW, Braddock-Wilking J, Rath NP (2017) Luminescent 2,7-disubstituted germafluorenes. J Organomet Chem 830:196–202
Zhao Y, Li X (2014) Detecting themicellization of anionic surfactants by a colorimetric and fluorescent probe based on electrostatic attraction. Colloid Polym Sci 292:1577–1584
Zhao Y, Li X (2015) Colorimetric and fluorometric detection of anionic surfactants withwater soluble sensors. Sens Actuators B Chem 209:258–264
Vasu A, Kanvah S (2017) Red-emitting cationic fluorophore as a probe for anionic surfactants. Dyes Pigm 142:230–236
Qian J, Qian X, Xu Y (2009) Selective and sensitive chromo- and fluorogenic dual detection of anionic surfactants in water based on a pair of “On–Off–On” fluorescent sensors. Chem Eur J 15:319–323
Mallick S, Arathi A, Koner AL (2017) Customized tuning of aggregation-induced emission of a napthalimide dye by surfactants and cyclodextrin. J Coll Interface Sci 499:46–53
Mallick S, Pal K, Koner AL (2016) Probing microenvironment of micelle and albumin using diethyl 6-(dimethylamino)naphthalene-2,3-dicarboxylate: An electroneutral solvatochromic fluorescent probe. J Coll Interface Sci 467:81–89
Li E, Lin L, Wang L, Pei M, Xu J, Zhang G (2012) Synthesis of a new cationic polythiophene derivative and its application for colorimetric and fluorometric detection of iodide ion and anionic surfactants in water. Macromol Chem Phys 213:887–892
Hussain S, Malik A, Iyer P (2015) Highly precise detection, discrimination, and removal of anionic surfactants over the full pH Range via cationic conjugated polymer: an efficient strategy to facilitate illicit-drug analysis. ACS Appl Mater Interfaces 7:3189–3198
Würth C, Grabolle M, Pauli J, Spieles M, Resch-Genger U (2013) Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat Protoc 8:1535–1550
Dhami S, de Mello AJ, Rumbles G, Bishop SM, Phillips D, Beeby A (1995) Phthalocyanine fluorescence at high concentration: dimers or reabsorption effect? Photochem Photobiol 61:341–346
Rurack K, Spieles M (2011) Fluorescence quantum yields of a series of red and near-infrared dyes emitting at 600 – 1000nm. Anal Chem 83:1232–1242
Long S, Qiao Q, Deng F, Miao L, Yoon J, Xu Z (2019) Self-assembling nanoprobes that display two-dimensional fluorescent signals for identification of surfactants and bacteria. Chem Commun 55:969–972
Stryer L (1968) Fluorescence spectroscopy of proteins. Science 162:526–533
Chen R (1967) Fluorescence quantum yields of tryptophan and tyrosine. Anal Lett 1:35–42
Jones I, Rahman G (1994) Fluorescence properties of coumarin laser dyes in aqueous polymer media. chromophore isolation in poly(methacrylic acid) hypercoils. J Phys Chem 98:13028–13037
Magde D, Rojas G, Seybold P (1999) Solvent dependence of the fluorescence lifetimes of xanthene dyes. Photochem Photobiol 70:737–744
Mujumdar RB, Ernst LA, Mujumdar SR, Lewis CJ, Waggoner AS (1993) Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters. Bioconjug Chem 4:105–111
Mohr A, Talbiersky P, Korth H-G, Sustmann R, Boese R, Blaser D, Rehage H (2007) A new pyrene-based fluorescent probe for the determination of critical micelle concentrations. J Phys Chem B 111:12985–12992
Klymchenko AS (2017) Solvatochromic and fluorogenic dyes as environment-sensitive probes: design and biological applications. Acc Chem Res 50:366–375
Sezgin E, Levental I, Mayor S, Eggeling C (2017) The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol 18:361–374. https://doi.org/10.1038/nrm.2017.16
Royer CA (2006) Probing protein folding and conformational transitions with fluorescence. Chem Rev 106:1769–1784
Algar WR, Hildebrandt N, Vogel SS, Medintz IL (2019) FRET as a biomolecular research tool - understanding its potential while avoiding pitfalls. Nat Methods 16:815–829
Funding
This work was supported by the National Science Foundation (to Janet Braddock-Wilking) CHE-1362431.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Helena Spikes, Shelby Jarrett-Noland and Stephan Germann. The first draft of the manuscript was written by Cynthia Dupureur and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2.71 MB)
Rights and permissions
About this article
Cite this article
Spikes, H.J., Jarrett-Noland, S.J., Germann, S.M. et al. Group 14 Metallafluorenes as Sensitive Luminescent Probes of Surfactants in Aqueous Solution. J Fluoresc 31, 961–969 (2021). https://doi.org/10.1007/s10895-021-02730-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-021-02730-3