Abstract
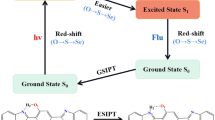
BODIPY fluorophores linked with an imidazo-phenanthroline donor at α and β positions have been synthesized. Intriguing intramolecular charge transfer phenomenon is observed in both the dyes which has been extensively investigated using UV–vis absorption, steady-state and time-resolved fluorescence measurements. H-bonding and intrinsic polarity of the solvents has modulated the absorption and emission bands of these fluorophores strongly causing significant increase in the Stokes shifts. In spite of having difference only in terms of the position of donor subunit, the photophysics of these dyes are not only significantly different from each other, but contradictory too. Interestingly, acidochromic studies revealed the shuttling mechanism between ICT and PET processes for BDP 2. Quantum chemical calculations have been employed further to support experimental findings. DFT and TD-DFT method of analysis have been used to optimize ground and excited state geometries of the synthesized dyes.







Similar content being viewed by others
References
Lu H, Mack J, Yang Y, Shen Z (2014) Structural modification strategies for the rational design of red/NIR region BODIPYs. Chem Soc Rev 43:4778–4823. https://doi.org/10.1039/C4CS00030G
Boens N, Leen V, Dehaen W (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41:1130–1172. https://doi.org/10.1039/C1CS15132K
Kamkaew A, Lim SH, Lee HB et al (2013) BODIPY dyes in photodynamic therapy. Chem Soc Rev 42:77–88. https://doi.org/10.1039/c2cs35216h
Bañuelos J (2016) BODIPY Dye, the most versatile fluorophore. Ever? Chem Rec 16:335–348. https://doi.org/10.1002/tcr.201500238
Ulrich G, Ziessel R, Harriman A (2008) The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew Chem Int Ed 47:1184–1201. https://doi.org/10.1002/anie.200702070
Ni Y, Wu J (2014) Far-red and near infrared BODIPY dyes: synthesis and applications for fluorescent pH probes and bio-imaging. Org Biomol Chem 12:3774–3791. https://doi.org/10.1039/c3ob42554a
Loudet A, Burgess K (2007) BODIPY dyes and their derivatives: syntheses and spectroscopic properties. Chem Rev 107:4891–4932. https://doi.org/10.1021/cr078381n
Alizadeh N, Akbarinejad A, Lu E et al (2015) Soluble fluorescent polymeric nanoparticles based on pyrrole derivatives: synthesis, characterization and their structure dependent sensing properties. J Mater Chem C 3:9910–9920. https://doi.org/10.1039/C5TC01982F
Jahanian MT, Akbarinejad A, Alizadeh N (2017) Design of a sensing platform with dual performance for detection of hydrogen peroxide and Fe3+ based on a new fluorescent oligo N-phenylpyrrole derivative. Sensors Actuators B Chem 240:971–978. https://doi.org/10.1016/j.snb.2016.09.078
Rurack K, Kollmannsberger M, Daub J (2001) A highly efficient sensor molecule emitting in the near infrared (NIR): 3,5-distyryl-8-(p-dimethylaminophenyl)difluoroboradiaza-s-indacene. New J Chem 25:289–292. https://doi.org/10.1039/b007379m
Wang D, Shiraishi Y, Hirai T (2010) A distyryl BODIPY derivative as a fluorescent probe for selective detection of chromium(III). Tetrahedron Lett 51:2545–2549. https://doi.org/10.1016/j.tetlet.2010.03.013
Banuelos-Prieto J, Agarrabeitia AR, Garcia-Moreno I et al (2010) Controlling optical properties and function of BODIPY by using asymmetric substitution effects. Chem Eur J 16:14094–14105. https://doi.org/10.1002/chem.201002095
Gai L, Mack J, Lu H et al (2014) New 2,6-distyryl-substituted BODIPY isomers: synthesis, photophysical properties, and theoretical calculations. Chem Eur J 20:1091–1102. https://doi.org/10.1002/chem.201303291
Zhu S, Zhang J, Vegesna G et al (2012) Controlled Knoevenagel reactions of methyl groups of 1,3,5,7-tetramethyl BODIPY dyes for unique BODIPY dyes. RSC Adv 2:404–407. https://doi.org/10.1039/c1ra00678a
Han J, Gonzalez O, Aguilar-Aguilar A et al (2009) 3- and 5-functionalized BODIPYs via the Liebeskind-Srogl reaction. Org Biomol Chem 7:34–36. https://doi.org/10.1039/b818390b
Richards GJ, Gobo Y, Yamamura M, Nabeshima T (2015) Biphenyl appended BODIPY derivatives showing combined environmental polarity and heavy metal cation sensing functionality. New J Chem 39:5886–5889. https://doi.org/10.1039/C5NJ00611B
Luo L, Wu D, Li W et al (2014) Regioselective decarboxylative direct C-H arylation of boron dipyrromethenes (BODIPYs) at 2,6-positions: a facile access to a diversity-oriented BODIPY library. Org Lett 16:6080–6083. https://doi.org/10.1021/ol502883x
Leen V, Leemans T, Boens N, Dehaen W (2011) 2- and 3-monohalogenated BODIPY dyes and their functionalized analogues: synthesis and spectroscopy. Eur J Org Chem 4386–4396. https://doi.org/10.1002/ejoc.201100324
Poronik YM, Yakubovskyi VP, Shandura MP et al (2010) 3,5-Bis(benzothiazolyl)-substituted BODIPY dyes. Eur J Org Chem 2746–2752. https://doi.org/10.1002/ejoc.201000084
Madhu S, Sharma DK, Basu SK et al (2013) Sensing Hg(II) in vitro and in vivo using a benzimidazole substituted BODIPY. Inorg Chem 52:11136–11145. https://doi.org/10.1021/ic401365x
Fang H-P, Wu Y-H, Lin H-C (2013) Synthesis and study of novel supramolecular nanocomposites containing aryl-imidazo-phenanthroline-based metallo-polymers (H-donors) and surface-modified ZnO nanoparticles (H-acceptors). Tetrahedron 69:293–301. https://doi.org/10.1016/j.tet.2012.10.031
Alreja P, Kaur N (2015) A new multifunctional 1, 10-phenanthroline based fluorophore for anion and cation sensing. J Lumin 168:186–191. https://doi.org/10.1016/j.jlumin.2015.08.010
Sangeetha S, Sathyaraj G, Muthamilselvan D et al (2012) Structurally modified 1,10-phenanthroline based fluorophores for specific sensing of Ni2+ and Cu2+ ions. Dalton Trans 41:5769–5773. https://doi.org/10.1039/c2dt30525a
Tao T, Wang SR, Chen L et al (2015) Synthesis and aggregation-induced emission of a pyrene decorated chiral BODIPY chromophore. Inorg Chem Commun 62:67–70. https://doi.org/10.1016/j.inoche.2015.10.029
Anzenbacher P, Tyson DS, Jursíková K, Castellano FN (2002) Luminescence lifetime-based sensor for cyanide and related anions. J Am Chem Soc 124:6232–6233. https://doi.org/10.1021/ja0259180
Yoldas A, Algi F (2015) An imidazo-phenanthroline scaffold enables both chromogenic Fe(II) and fluorogenic Zn(II) detection. RSC Adv 5:7868–7873. https://doi.org/10.1039/C4RA14182B
Ziessel R, Bonardi L, Retailleau P, Ulrich G (2006) Isocyanate-, isothiocyanate-, urea-, and thiourea-substituted boron dipyrromethene dyes as fluorescent probes. J Org Chem 71(8):3093–3102. https://doi.org/10.1021/jo0600151
Filarowski A, Lopatkova M, Lipkowski P et al (2015) Solvatochromism of BODIPY-schiff dye. J Phys Chem B 119:2576–2584. https://doi.org/10.1021/jp508718d
Haefele A, Zedde C, Retailleau P et al (2010) Boron asymmetry in a BODIPY derivative. Org Lett 12:1672–1675. https://doi.org/10.1021/ol100109j
Jacobsen JA, Stork JR, Magde D, Cohen SM (2010) Hydrogen-bond rigidified BODIPY dyes. Dalton Trans 39:957–962. https://doi.org/10.1039/b921772j
Lauderdale WJ, Coolidge MB (1995) Basis set effects on the nonlinear optical properties of selected linear diacetylenes. J Phys Chem 99:9368–9373. https://doi.org/10.1021/j100023a011
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255:327–335. https://doi.org/10.1016/0009-2614(96)00349-1
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093. https://doi.org/10.1021/cr9904009
Kothavale S, Sekar N (2017) Novel pyrazino-phenanthroline based rigid donor-π-acceptor compounds: a detail study of optical properties, acidochromism, solvatochromism and structure-property relationship. Dye Pigment 136:31–45. https://doi.org/10.1016/j.dyepig.2016.08.032
Goswami S, Das AK, Maity S (2013) “PET” vs. “push-pull” induced ICT: a remarkable coumarinyl-appended pyrimidine based naked eye colorimetric and fluorimetric sensor for the detection of Hg2 + ions in aqueous media with test trips. Dalton Trans 42:16259–16263. https://doi.org/10.1039/c3dt52252k
Panchenko PA, Fedorov YV, Fedorova OA, Jonusauskas G (2013) Comparative analysis of the PET and ICT sensor properties of 1,8-naphthalimides containing aza-15-crown-5 ether moiety. Dye Pigment 98:347–357. https://doi.org/10.1016/j.dyepig.2013.03.008
Wei T, Wang J, Chen Y, Han Y (2015) Combining the PeT and ICT mechanisms into one chemosensor for the highly sensitive and selective detection of zinc. RSC Adv 5:57141–57146. https://doi.org/10.1039/C5RA11194C
Coskun A, Deniz E, Akkaya EU (2005) Effective PET and ICT switching of boradiazaindacene emission: a unimolecular, emission-mode, molecular half-subtractor with reconfigurable logic gates. Org Lett 7:5187–5189. https://doi.org/10.1021/ol052020h
Bozdemir OA, Guliyev R, Buyukcakir O et al (2010) Selective manipulation of ICT and PET processes in styryl-bodipy derivatives: applications in molecular logic and fluorescence sensing of metal ions. J Am Chem Soc 132:8029–8036. https://doi.org/10.1021/ja1008163
Choi H, Lee JH, Jung JH (2014) Fluorometric/colorimetric logic gates based on BODIPY-functionalized mesoporous silica. Analyst 139:3866–3870. https://doi.org/10.1039/c4an00251b
Acknowledgements
Authors thank Dr. H. Pal of Radiation & Photochemistry Division, BARC, Mumbai for allowing fluorescence lifetime decay measurements of dye samples in his lab. Author S.T. thanks DAE-BRNS for a Senior Research Fellowship. AKR and NS acknowledges DAE-BRNS for supporting this collaboration research.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Additional information about synthesis, characterization, fluorescence decay curves and TDDFT results are provided.
Rights and permissions
About this article
Cite this article
Thakare, S.S., Chakraborty, G., Kothavale, S. et al. Proton Induced Modulation of ICT and PET Processes in an Imidazo-phenanthroline Based BODIPY Fluorophores. J Fluoresc 27, 2313–2322 (2017). https://doi.org/10.1007/s10895-017-2173-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-017-2173-4