Abstract
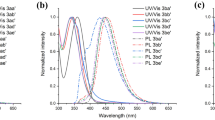
A new series of bodipy-functionalized cyclotriphosphazene derivatives were designed and synthesized. The identities of all newly synthesized compounds were confirmed by using 1H, 13C, 31P NMR spectroscopies. The photophysical properties of bodipy-functionalized cyclotriphosphazenes were investigated via absorption and fluorescense spectroscopies in dichloromethane. Singlet oxygen generation capacities of new compounds were also examined using the trap molecule 1,3-diphenylisobenzofuran. The targeted compounds showed high molar extinction coefficients in the NIR region and respectable singlet oxygen quantum yields when compared to that of methylene blue. The new bodipy-functionalized cyclotriphosphazenes are efficient photosensitizers to be potentially used for the singlet oxygen generation.





Similar content being viewed by others
References
DeRosa MC, Crutchley RJ (2002) Photosensitized singlet oxygen and its applications. Coord Chem Rev 233–234:351–371
Kim H, Kim W, Mackeyev Y, Lee GS, Kim HJ, Tachikawa T, Hong S, Lee S, Kim J, Wilson LJ, Majima T, Alvarez PJJ, Choi W, Lee J (2012) Selective oxidative degradation of organic pollutants by singlet oxygen-mediated photosensitization: Tin Porphyrin versus C60Aminofullerene systems. Environ Sci Technol 46:9606–9613
Sharman WM, Allen GM, VanLier JE (1999) Photodynamic therapeutics: basic principles and clinical applications. Drug Discov Today 4:507–517
Agnez-Lima LF, Melo JTA, Silva AE, Oliveira AHS, Timoteo ARS, Lima-Bessa KM, Martinez GR, Medeiros MHG, Mascio PD, Galhardo RS, Menck CFM (2012) DNA damage by singlet oxygen and cellular protective mechanisms. Mutat Res 751:15–28
Kamkaew A, Lim SH, Lee HB, Kiew LV, Chungcand LY, Burgess K (2013) BODIPY dyes in photodynamic therapy. Chem Soc Rev 42:77–88
Bonnett R (1995) Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem Soc Rev 24:19–33. doi:10.1039/CS9952400019
Lovell JF, Liu TWB, Chenand J, Zheng G (2010) Activatable photosensitizers for imaging and therapy. Chem Rev 110:2839–2857
Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW, Hasan T (2010) Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev 110:2795–2838
Atilgan S, Ekmekci Z, Dogan AL, Guc D, Akkaya EU (2006) Water soluble distyryl-boradiazaindacenes as efficient photosensitizers for photodynamic therapy. Chem Commun 4398–4400. doi:10.1039/B612347C
Cakmak Y, Kolemen S, Duman S, Dede Y, Dolen Y, Kilic B, Kostereli Z, Tatar Yildirim L, Dogan AL, Guc D, Akkaya EU (2011) Designing excited states: theory-guided access to efficient photosensitizers for photodynamic action. Angew Chem Int Ed 50:11937–11941
Yogo T, Urano Y, Ishitsuka Y, Maniwaand F, Nagano T (2005) Highly efficient and photostable photosensitizer based on BODIPY chromophore. J Am Chem Soc 127:12162–12163
Awuahaband SG, You Y (2012) Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy. RSC Adv 2:11169–11183
Zhao J, Wu W, Sun J, Guo S (2013) Triplet photosensitizers: from molecular design to applications. Chem Soc Rev 42:5323–5351
Zhao J, Xu K, Yang W, Wang Z, Zhong F (2015) The triplet excited state of Bodipy: formation, modulation and application. Chem Soc Rev 44:8904–8939
Adarsh N, Avirah RR, Ramaiah D (2010) Tuning photosensitized singlet oxygen generation efficiency of novel Aza-BODIPY dyes. Org Lett 12(20):5720–5723
Rao MR, Bolligarla R, Butcherand RJ, Ravikanth M (2010) Hexa boron-dipyrromethene cyclotriphosphazenes: synthesis, crystal structure, and photophysical properties. Inorg Chem 49:10606–10616
Cosut B, Hacıvelioglu F, Durmuş M, Kılıç A, Yesilot S (2009) The synthesis, thermal and photophysical properties of phenoxycyclotriphosphazenyl-substituted cyclic and polymeric phosphazenes. Polyhedron 28:2510–2516
Çiftçi GY, Şenkuytu E, Durmuş M, Yuksel F, Kılıç A (2013) Fluorenylidene bridged cyclotriphosphazenes: ‘turn off’ fluorescence probe for Cu2+ and Fe3+ ions. Dalton Trans 42:14916–14926
Şenkuytu E, Eçik ET, Durmuş M, Çiftçi GY (2015) Monofunctional amines substituted fluorenylidene bridged cyclotriphosphazenes: ‘Turn-off’ fluorescence chemosensors for Cu2+ and Fe3+ ions. Polyhedron 101:223–229
Kagit R, Yildirim M, Ozay O, Yesilot S, Ozay H (2014) Phosphazene based multicentered naked-eye fluorescent sensor with high selectivity for Fe3+ ions. Inorg Chem 53(4):2144–2151
Yıldırım T, Bilgin K, Çiftçi GY, Eçik ET, Şenkuytu E, Uludağ Y, Tomak L, Kılıç A (2012) Synthesis, cytotoxicity and apoptosis of cyclotriphosphazene compounds as anti-cancer agents. Eur J Med Chem 52:213–220
Siwy M, Seük D, Kaczmarczyk B, Jaroszewicz I, Nasulewicz A, Pelczynska M, Nevozhayand D, Opolski A (2006) Synthesis and in vitro antileukemic activity of some new 1,3-(oxytetraethylenoxy)cyclotriphosphazene derivatives. J Med Chem 49:806–810
Allen CW (1991) Regie and stereochemical control in substitution reactions of cyclophosphazenes. Chem Rev 91:119–135
Chandrasekharand V, Nagendran S (2001) Phosphazenes as scaffolds for the construction of multi-site coordination ligands. Chem Soc Rev 30:193–203
Caminade AM, Hameauaand A, Majorala JP (2016) The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans 45:1810–1822
Rao MR, Gayatri G, Kumar A, Sastryand GN, Ravikanth M (2009) Cyclotriphosphazene ring as a platform for multiporphyrin assemblies. Chem Eur J 15:3488–3496
Franc G, Maze`res S, Turrin CO, Vendier L, Duhayon C, Caminadeand AM, Majoral JP (2007) Synthesis and properties of dendrimers possessing the same fluorophore(s) located either peripherally or off-center. J Org Chem 72:8707–8715
Çiftçi GY, Eçik ET, Bulut M, Yuksel F, Kılıç A, Durmus M (2013) Synthesis and characterization of dicoumarol substituted cyclotriphosphazenes. Inorg Chim Acta 398:106–112
Cosut B (2014) Highly efficient energy transfer in BODIPY-pyrene decorated Cyclotriphosphazene. Dyes Pigments 100:11–16
Çiftçi GY, Şenkuytu E, Incir SA, Yuksel F, Olcer Z, Yildirim T, Kılıç A, Uludag Y (2016) First paraben substituted cyclotetraphosphazene compounds and DNA interaction analysis with a new automated biosensor. Biosens Bioelectron 80:331–338
Eçik ET, Şenkuytu E, Cebesoy Z, Çiftçi GY (2016) BODIPY decorated dendrimeric cyclotriphosphazene photosensitizers: synthesis and efficient singlet oxygen generators. RSC Adv 6:47600–47606
Acknowledgments
The authors thank the Scientific and Technical Research Council of Turkey for financial support TUBITAK (Grant 114Z445).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2126 kb)
Rights and permissions
About this article
Cite this article
Şenkuytu, E., Cebesoy, Z., Çiftçi, G.Y. et al. Study on the Synthesis, Photophysical Properties and Singlet Oxygen Generation Behavior of Bodipy-Functionalized Cyclotriphosphazenes. J Fluoresc 27, 595–601 (2017). https://doi.org/10.1007/s10895-016-1987-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1987-9