Abstract
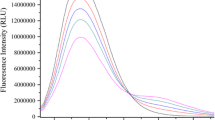
The interactions between uranium and two functional proteins (AChE and Vtg) were investigated using fluorescence quenching measurements. The combined use of a microplate spectrofluorometer and logarithmic additions of uranium into protein solutions allowed us to define the fluorescence quenching over a wide range of [U]/[Pi] ratios (from 1 to 3235) at physiologically relevant conditions of pH. Results showed that fluorescence from the two functional proteins was quenched by UO2 2+. Stoichiometry reactions, fluorescence quenching mechanisms and complexing properties of proteins, i.e. binding constants and binding sites densities, were determined using classic fluorescence quenching methods and curve-fitting software (PROSECE). It was demonstrated that in our test conditions, the protein complexation by uranium could be simulated by two specific sites (L1 and L2). The obtained complexation constant values are log K1 = 5.7 (±1.0), log K2 = 4.9 (±1.1); L1 = 83 (±2), L2 = 2220 (±150) for U(VI) – Vtg and log K1 = 8.1 (±0.9), log K2 = 6.6 (±0.5), L1 = 115 (±16), L2 = 530 (±23) for U(VI)-AChE (Li is expressed in mol/mol of protein).




Similar content being viewed by others
References
Colle C, Garnier-Laplace J, Roussel-Debet S, Adam C, Baudin JP (2001) Comportement de l’uranium dans l’environnement. In: Métivier H (Ed.) L’uranium, de l’environnement à l’homme, EDP Sci., Les Ullis, pp. 187–211.
Van Horn JD, Huang H (2006) Uranium(VI) bio-coordination chemistry from biochemical, solution and protein structural data. Coord Chem Rev 250(7–8):765–775
Vidaud C, Dedieu A, Basset C, Plantevin S, Dany I, Pible O, Quéméneur E (2005) Screening of human serum proteins for uranium binding. Chem Res Toxicol 18(6):946–953
Taylor DM (1998) The bioinorganic chemistry of actinides in blood. J Alloys Compd 271–273:6–10
Ansoborlo E, Prat O, Moisy P, Den Auwer C, Guilbaud P, Carrière M, Gouget B, Duffield J, Doizy D, Vercouter T, Moulin C, Moulin V (2006) Actinide speciation in relation to biological processes. Biochimie 88(11):1605–1618
Michon J, Frelon S, Garnier C, Coppin F (2010) Determination of uranium(VI) binding properties with some metalloproteins (transferrin, albumin, metallothionein and ferritin) by fluorescence quenching. J Fluoresc 20:581–590
Pandey S, Ali M, Bishnoi A, Azam A, Pandey S, Chawla HM (2008) Quenching of pyrene fluorescence by calix[4]arene and calix[4]resorcinarenes. J Fluoresc 18(2):533–539
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, Berlin Heidelberg New-York
Valeur B (2007) Molecular fluorescence: principles and applications 4th reprint. Wiley-VCH, Weinheim
Barillet S, Adam C, Palluel O, Devaux A (2007) Bioaccumulation, oxidative stress, and neurotoxicity in Danio rerio exposed to different isotopic composition of uranium. Environ Toxicol Chem 26(3):497–505
Bourrachot S, Simon O, Gilbin R (2008) The effects of waterborne uranium on the hatching success, development, and survival of early life stages of zebrafish (Danio rerio). Aquat Toxicol 90(1):29–36
Lestaevel P, Bensoussan H, Dhieux B et al (2013) Cerebral cortex and hippocampus respond differently after post-natal exposure to uranium. J Toxicol Sci 38(5):803–811
Locatello L, Matozzo V, Marin MG (2009) Biomarker responses in the crab Carcinus aestuarii to assess environmental pollution in the Lagoon of Venice (Italy). Ecotoxicology 18(7):869–877
Guedes RNC, Zhu KY, Kambhampati S, Dover BA (1998) Characterization of acetylcholinesterase purified from the lesser grain borer, rhyzopertha dominica (coleoptera: bostrichidae). Comp Biochem Physiol C 119(2):205–210
Rajesh RV, Balasubramanian AS, Boopathy R (2009) Evidence for presence of Zn2+-binding site in acetylcholinesterase. Biochimie 91(4):526–532
Quinn DM (1987) Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition state. Chem Rev 87(5):955–979
Tõugu V, Kesvatera T (2001) Comparison of salts effects on the reactions of acetylcholinesterase with cationic and anionic inhibitors. Biochim Biophys Acta 1544(1–2):189–195
Brion F, Nielsen BM, Eidem JK, Goksøyr A, Porcher JM (2002) Development and validation of an enzyme-linked immunosorbent assay to measure vitellogenin in the zebrafish (Danio rerio). Environ Toxicol Chem 28(8):1699–1708
Hwang UG, Kagawa N, Mugiya Y (2000) Aluminium and cadmium inhibit vitellogenin and its mRNA induction by estradiol-17 b in the primary culture of hepatocytes in the rainbow trout Oncorhynchus mykiss. Gen Comp Endocrinol 119(1):69–76
Garnier C, Pižeta I, Mounier S, Benaim JY, Branica M (2004) Influence of the type of titration and of data treatment methods on metal complexing parameters determination of single- and multi-ligand systems measured by stripping voltammetry. Anal Chim Acta 505(2):263–275
Louis L, Garnier C, Lenoble V, Omanović D, Mounier S, Pižeta I (2009) Characterisation and modelling of marine dissolved organic matter interactions with major and trace cations. Mar Environ Res 67(2):100–107
Lenoble V, Garnier C, Masion A, Ziarelli F, Garnier JM (2008) Combination of 13C/113Cd NMR, potentiometry, and voltammetry in characterizing the interactions between Cd and two models of the main components of soil organic matter. Anal Bioanal Chem 390(2):749–757
Radić Z, Kim E, Taylor P (2004) Intrinsic Tryptophan Fluorescence of Cholinesterases: Direct, Nonperturbing Monitoring of Enzyme-Ligand Interactions. In: Silman I, Soreq H, Anglister L, Michaelson D, Fisher A (Eds.), Cholinergic mechanisms, function and dysfunction. Abingdon, pp. 171–174
Komatsu M, Matsumoto W, Hayashi S (1996) Protease activity appeared after trypsin treatment of the purified vitellogenin from eel Anguilla japonica. Comp Biochem Physiol B 113(3):561–571
Scapolan S, Ansoborlo E, Moulin C, Madic C (1998) Uranium (VI)-transferrin system studied by time-resolved laser-induced fluorescence. Radiat Prot Dosim 79(1–4):505–508
Gök E, Öztürk C, Akbay N (2008) Interaction of thyroxine with 7 hydroxycoumarin: a fluorescence quenching study. J Fluoresc 18(5):781–785
Keppler JK, Stuhldreier MC, Temps F, Schwarz K (2014) Influence of mathematical models and correction factors on binding results of polyphenols and retinol with β-lactoglobulin measured with fluorescence quenching. Food Biophys 9(2):158–168
Omanović D, Garnier C, Pižeta I (2015) ProMCC: an all-in-one tool for trace metal complexation studies. Mar Chem. doi:10.1016/j.marchem.2014.10.011
Wiley HS, Wallace RA (1981) The structure of vitellogenin. Multiple vitellogenins in Xenopus laevis give rise to multiple forms of the yolk proteins. J Biol Chem 256(16):8626–8634
Castellani O, Guérin-Dubiard C, David-Briand E, Anton M (2004) Influence of physicochemical conditions and technological treatments on the iron binding capacity of egg yolk phosvitin. Food Chem 85(4):569–577
Grbac-Ivankovic S, Antonijczuk K, Varghese AH et al (1994) Lipovitellin 2β is the 31 kD Ni2+−binding protein (pNiXb) in Xenopus oocytes and embryos. Mol Reprod Dev 38(3):256–263
Sunderman FW Jr, Antonijczuk K, Antonijczuk A, Grbac-Ivankovic S, Varghese AH, Korza G, Ozols J (1995) Xenopus lipovitellin 1 is a Zn2+− and Cd2+− binding protein. Mol Reprod Dev 42(2):180–187
Choi I, Jung C, Choi H, Kim C, Ha H (2005) Effectiveness of phosvitin peptides on enhancing bioavailability of calcium and its accumulation in bones. Food Chem 93(4):577–583
Pardoux R, Sauge-Merle S, Lemaire D, Delangle P, Guilloreau L, Adriano JM, Berthomieu C (2012) Modulating uranium binding affinity in engineered calmodulin EF-hand peptides: effect of phosphorylation. PLoS ONE 7(8):e41922
Safi S, Creff G, Jeanson A et al (2013) Osteopontin: a uranium phosphorylated binding-site characterization. Chem Eur J 19(34):11261–11269
Li B, Raff J, Barkleit A, Bernhard G, Foerstendorf H (2010) Complexation of U(VI) with highly phosphorylated protein, phosvitin. A vibrational spectroscopic approach. J Inorg Biochem 104(7):718–725
Ghosh P, Thomas P (1995) Binding of metals to red drum vitellogenin and incorporation into oocyte. Mar Environ Res 39(1–4):165–168
Martín-Díaz ML, Bamber S, Casado-Martínez C, Sales D, DelValls TA (2004) Toxicokinetics of heavy metals from a mining spill using Carcinus maenas. Mar Environ Res 58(2–5):833–837
Acknowledgments
Thanks to INERIS (Institut National de l’Environnement Industriel et des Risques, Verneuil-en-Hallate, France) that provides the Vtg.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(DOC 82 kb)
Rights and permissions
About this article
Cite this article
Coppin, F., Michon, J., Garnier, C. et al. Fluorescence Quenching Determination of Uranium (VI) Binding Properties by Two Functional Proteins: Acetylcholinesterase (AChE) and Vitellogenin (Vtg). J Fluoresc 25, 569–576 (2015). https://doi.org/10.1007/s10895-015-1536-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1536-y