Abstract
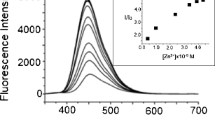
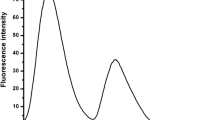
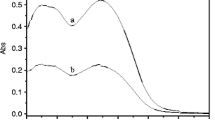
The fluorescence intensity of salicylaldehyde phenylhydrazone (L), in 1:1 (v/v) CH3OH:H2O was enhanced by ca. 100 times with a blue shift in emission maximum, on interaction with Pb2+ ion. No enhancement in fluorescent intensity of L was observed on interaction with metal ions - Na+, K+, Ca2+, Cu2+, Ni2+, Zn2+, Cd2+ and Hg2+. This signal transduction was found to occur via photoinduced electron transfer (PET) mechanism. A 1:1 complexation between Pb2+ and L with log β = 7.86 has been proved from fluorescent and UV/Visible spectroscopic data. The detection limit of Pb2+ was calculated to be 6.3 × 10−7 M.







Similar content being viewed by others
References
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40
Prodi L, Bolletta L, Montalti M, Zaccheroni N (2000) Luminescent chemosensors for transition metal ions. Coord Chem Rev 205:59–83
Yoosaf K, Ipe BI, Suresh CH, Thomas KG (2007) In situ synthesis of metal nanoparticles and selective naked-eye detection of lead ions from aqueous media. J Phys Chem C 111:12839–12847
Ragan P, Turner T (2009) Working to prevent lead poisoning in children: getting the lead out. J Am Acad Phys Asst 22:40–45
Needleman HL (1992) Human lead exposure. CRC Press, Boca Raton
Florin TA, Brent TMW, Weitzman M (2005) The need for vigilance: the persistence of lead poisoning in children. Pediatrics 115:1767–1768
Garza A, Vega R, Soto E (2006) Cellular mechanisms of lead neurotoxicity. Med Sci Monit 12:RA57–RA65
Magyar JS, Weng TC, Stern CM, Dye DF, Rous BW, Payne JC, Bridgewater BM, Mijovilovich A, Parkin G, Zaleski JM, Penner-Hahn JE, Godwin HA (2005) Reexamination of lead(II) coordination preferences in sulfur-rich sites: implications for a critical mechanism of lead poisoning. J Am Chem Soc 127:9495–9505
Abbate C, Buceti R, Munao F, Giorgianni C, Ferreri G (1995) Neurotoxicity induced by lead levels: an electrophysiological study. Int Arch Occup Environ Health 66:389–392
Manahan SE (1994) Environmental chemistry. CRC Press, Boca Raton
Basabe-Desmonts L, Reinhoudt DN, Crego-Calama M (2007) Design of fluorescent materials for chemical sensing. Chem Soc Rev 36:993–1017
Valeur B, Leray I (2007) Ion-responsive supramolecular fluorescent systems based on multichromophoric calixarenes: a review. Inorg Chim Acta 360:765–774
Jiang P, Guo Z (2004) Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors. Coord Chem Rev 248:205–229
Prodi L (2005) Luminescent chemosensors: from molecules to nanoparticles. New J Chem 29:20–31
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205:3–40
Métivier R, Leray I, Valeur B (2003) A highly sensitive and selective fluorescent molecular sensor for Pb (II) based on a calix [4] arene bearing four dansyl groups. Chem Commun 8:996–997
de Silva AP, Fox DB, Huxley AJM, Moody TS (2000) Combining luminescence, coordination and electron transfer for signalling purposes. Coord Chem Rev 205:41–57
Zhang J, Campbell RE, Ting AY, Tisen RY (2002) Creating new fluorescent probes for cell biology. Nat Rev Mol Cell Biol 3:906–918
Yao J, Li J, Owens J, Zhong W (2011) Combing DNAzyme with single-walled carbon nanotubes for detection of Pb(II) in water. Analyst 136:764–768
Goswami P, Das DK (2012) N, N, N, N-tetradentate macrocyclic ligand based selective fluorescent sensor for zinc (II). J Fluorescence 22:1081–1085
Gong Z-L, Zhao B-X, Liu W-Y, Lv H-S (2011) A new highly selective “turn-on” fluorescent sensor for zinc ion based on a pyrazoline derivative. J Photochem Photobiol A 218:6–10
Goswami P, Baruah S, Das DK (2010) 2,7-dichlorofluorescein, a fluorescent sensor to detect Cd2+ over Na+, K+, Ca2+, Cu2+, Ni2+ and Zn2+. Indian J Chem A 49:1617–1620
Goswami P, Das DK (2012) A new highly sensitive and selective fluorescent cadmium sensor. J Fluorescence 22:391–395
Jiang J, Liu W, Cheng J, Yang L, Jiang H, Bai D, Liu W (2012) A sensitive colorimetric and ratiometric fluorescent probe for mercury species in aqueous solution and living cells. Chem Comm 48:8371–8373
Yan F, Cao D, Wang M, Yang N, Yu Q, Dai L, Chen L (2012) A new rhodamine-based “off-on” fluorescent chemosensor for Hg(II) ion and its application in imaging Hg(II) in living cells. J Fluorescence 22:1249–1256
Fan J, Peng X, Wang S, Liu X, Li H, Sun S (2012) A fluorescence turn-on sensor for Hg2+ with a simple receptor available in sulphide-rich environments. J Fluorescence 22:941–955
Liu J, Yu M, Wang X-C, Zhang Z (2012) A highly selective colorimetric sensor for Hg2+ based on nitrophenyl-aminothiourea. Spectrochim Acta Part A 93:245–249
Deo S, Godwin HA (2000) A selective, ratiometric fluorescent sensor for Pb2+. J Am Chem Soc 122:174–175
Liu J, Yi L (2003) A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J Am Chem Soc 125:6642–6643
Hou C, Xiong Y, Fu N, Jacquot CC, Squier TC, Cao H (2011) Turn-on ratiometric fluorescent sensor for Pb2+ detection. Tetrahedron Lett 52:2692–2696
Sun M, Shangguan D, Ma H, Nie L, Li X, Xiong S, Liu G, Thiemann W (2003) Simple Pb II fluorescent probe based on PbII-catalyzed hydrolysis of phosphodiester. Biopolymers 72:413–420
Aksuner N (2011) Development of a new fluorescent sensor based on a triazolo-thiadiazin derivative immobilized in polyvinyl chloride membrane for sensitive detection of lead(II) ions. Sensors Actuators B 157:162–168
Ma L, Li H, Wu Y (2009) A pyrene-containing fluorescent sensor with high selectivity for lead (II) ion in water with dual illustration of ground-state dimer. Sensors Actuators B 143:25–29
Kwon JY, Jang YJ, Lee YJ, Kim KM, Seo MS, Nam W, Yoon J (2005) A highly selective fluorescent chemosensor for Pb2+. J Am Chem Soc 127:10107–10111
Zapata F, Caballero A, Espinosa A, Tárraga A, Molina P (2009) Imidazole-annelated ferrocene derivatives as highly selective and sensitive multichannel chemical probes for Pb(II) cations. J Org Chem 74:4787–4796
Love BE, Jones EG (1999) The use of salicylaldehyde phenylhydrazone as an indicator for the titration of organometallic reagents. J Org Chem 64:3755–3756
Dong X, Yang Y, Sun J, Liu Z, Bi-F L (2009) Two -photonexcited fluorescent probes for calcium based on internal chargetransfer. Chem Commun 26:3883–3885
Ashokkumar P, Ramakrishnan VT, Ramamurthy P (2011) Photoinduced Electron Transfer (PET) based Zn2+ fluorescent probe: transformation of turn-on sensors into ratiometric ones with dual emission in acetonitrile. J Phys Chem A 2011:14292–14299
Bryan AJ, de Silva AP, Rupasinghe RADD, Sadayake KRAS (1989) Photo-induced electron transfer as a general design logic for fluorescent molecular sensors for cations. Biosensors 4:169–179
Goldstein GW (1993) Evidence that lead acts as a calcium substitute in secondmessenger metabolism. Neurotoxicology 14:97–102
Kulatilleke CP, Silva SA, Eliav Y (2006) A coumarin based fluorescent photoinduced electron transfer cation sensor. Polyhedron 25:2593–2596
Acknowledgment
UGC, New Delhi and DST, New Delhi are thanked for financial support to the Department. PG thanks the former for fellowship under RFSMS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, D.K., Goswami, P. & Sarma, S. Salicylaldehyde Phenylhydrazone: A New Highly Selective Fluorescent Lead (II) Probe. J Fluoresc 23, 503–508 (2013). https://doi.org/10.1007/s10895-013-1167-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-013-1167-0