Abstract
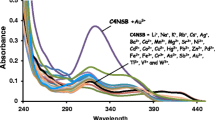
Novel Supramolecular fluorescence receptor derived from calix-system i.e. calix[4]resorcinarene bearing dansylchloride as fluorophore was designed and synthesized. The compound was purified by column chromatography and characterized by elemental analysis, NMR and Mass spectroscopy. Tetradansylated calix[4] resorcinarene (TDCR) shows a boat conformation with C2v symmetry. The complexation behaviour of metal cations [Ag(I), Cd(II), Co(II), Fe(III), Hg(II), Cu(II), Pb(II), Zn(II), U(VI) (1 × 10-4 M)] with tetra dansylated calix[4]resorcinarene (1 × 10-6 M) was studied by spectophotometry and spectrofluorometry. Red shift in the absorption spectra led us to conclude that there is strong complexation Fe(III), Co(II) and Cu(II) with TDCR. These metal cations also produce quenching with red shifts in the emission spectra. The maximum quenching in emission intensity was observed in the case of Fe(III) and its binding constant was also found to be significantly higher than that of Co(II) and Cu(II). Quantum yield of metal complexes of Fe(III) was found to be lower in comparison with Co(II) and Cu(II) complexes. Stern Volmer analysis indicates that the mechanism of fluorescence quenching is either purely dynamic, or purely static.







Similar content being viewed by others
References
Atood JL, Davies JED, MacNicol DD, Vogetle F (1996) Comprehensive Supramolecular Chemistry, ed.; Elsevier: Exeter
De Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97:1515–1566
Lee SH, Kim SH, Kim SK, Jung JH, Kim JS (2005) Bifunctional fluorescent Calix[4]arene chemosensor for both a cation and an anion. J Org Chem 70:1463–1466
Hamdi A, Kim SH, Abidi R, Thuery P, Kim JS, Vicens J (2009) A dipyrenyl calixazacrown chemosensor for Mg2+. Tetrahedron 65:2818–2823
Anger P, Bharadwaj P, Novotny L (2006) Enhancement and quenching of single-molecule fluorescence. PRL 96:113002-1-4
Lakowicz JR, Weber G (1973) Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 12(21):4161–4170
Guo X, Qian X, Jia L (2004) A highly selective and sensitive fluorescent chemosensor for Hg2+ in neutral buffer aqueous solution. J Am Chem Soc 126(8):2272–2273
Aragoni MC, Arca M, Bencini A, Blake AJ, Caltagirone C, Danesi A, Devillanova FA, Garau A, Gelbrich T, Isaia F, Lippolis V, Hursthouse MB, Valtancoli B, Wilson C (2007) New fluorescent chemosensors for heavy metal ions based on functionalized pendant arm derivatives of 7-anthracenylmethyl-1,4,10-trioxa-7,13-diazacyclopentadecane. Inorg Chem 46:8088–8097
Misra A, Shahid M, Srivastava P, Dwivedi P (2010) Fluorescent chemosensor: recognition of metal ions in aqueous medium by fluorescence quenching. J Inclusion Phenom Macrocylic Chem 69:119–129
Chen QY, Chen CF (2005) A new Hg2+ selective fluorescent sensor based on a dansyl amide-armed calix[4]-aza-crown. Tetrahedron Lett 46:165–168
Talanova GG, Elkarim NSA, Talanov VS, Bartsch RA (1999) A calixarene-based fluorogenic reagent for selective mercury(II) recognition. Anal Chem 71:3106–13109
Talanova GG, Roper ED, Buie NM, Gorbunova MG, Bartsch RA, Talanov VS (2005) Novel fluorogenic calix[4]arene-bis(crown-6-ether) for selective recognition of thallium(I), Chem. Commun. 5673–5675
Schonefeld K, Ludwig R, Feller KH (2006) Fluorescence studies of host–guest interaction of a dansyl amide labelled Calix[6]arene. J Fluoresc 16:449–454
Fusi V, Giorgi L, Micheloni M (2012) New fluorescent chemosensors for metal ions in solution Mauro Formica. Coord Chem Rev 256:170–192
David Gutsche C (2008) Calixarenes: an introduction, 261-276, Ed. RSC publication
Kim JS, Lee SY, Yoon J, Vicens J (2009) Hyperbranched calixarenes: synthesis and applications as fluorescent probes. Chem Commun 4791–4802
Kim JS, Quang DT (2007) Calixarene-derived fluorescent probes. Chem Rev 107(9):3780–3799
Sahin O, Yilmaz M (2011) Synthesis and fluorescence sensing properties of novel pyrene-armed calix[4]arene derivatives. Tetrahedron 67:3501–3508
Roberts BA, Cave GWV, Raston CL, Scott JL (2001) Solvent-free synthesis of calix[4]resorcinarenes. Green Chemistry 3:280–284
Mclldowie MJ, Mocerino M, Ogden MI (2010) A brief review of C-symmetric calixarenes and resorcinarenes. Supramol Chem 22(1):13–39
Pandey S, Ali M, Bishnoi A, Azam A, Pandey S, Chawla HM (2008) Quenching of pyrene fluorescence by Calix[4]arene and Calix[4]resorcinarenes. J Fluoresc 18:533–539
Fix OK, Kowdley KV (2008) Hereditary hemochromatosis. Minerva Med 99:605–617
Li CY, Zhang XB, Jin Z, Han R, Shen GL, Yu RQ (2006) A fluorescent chemosensor for cobalt ions based on a multi-substituted phenol-ruthenium(II) tris(bipyridine) complex. Anal Chim Acta 580:143–148
Santander PJ, Kajiwara Y, Williams HJ, Scott AI (2006) Structural characterization of novel cobalt corrinoids synthesized by enzymes of the vitamin B12 anaerobic pathway. Bioorg Med Chem 14:724–731
Frank A, McPartlin J, Danielsson R (2004) Nova Scotia moose mystery-a moose sickness related to cobalt- and vitamin B12 deficiency. Sci Total Environ 318:89–100
Al-Habsi K, Johnson EH, Kadim IT, Srikandakumar A, Annamalai K, Al-Busaidy R, Mahgoub O (2007) Effects of low concentrations of dietary cobalt on liveweight gains, haematology, serum vitamin B(12) and biochemistry of Omani goats. Vet J 173:131–137
Da Silva JJRF, Williams RJP (1991) The biological chemistry of the elements. Clarendon, Oxford
Hogberg AGS (1980) Stereoselective synthesis and DNMR study of two 1,8,15,22-Tetraphenyl [14] metacyclophan-3,5,10,12,17,19,24,26-octols. J Am Chem Soc 102:6046–6050
Hogberg AGS (1980) Two steroisomeric macrocyclic resorcinol-acetaldehyde condensation products. J Org Chem 45:4498–4500
Timmerman P, Verboom W, Reinhoudt DN (1996) Resorcinarenes. Tetrahedron 52:2663–2704
Beyeh NK, Aumanen J, Ahman A, Luostarinen M, Mansikkamaki H, Nissinen M, Tommola JK, Rissanen K (2007) Dansylated resorcinarenes. New J Chem 31:370–376
Li YH, Chan LM, Tyer L, Moody RT, Himel CM, Hercules DM (1975) Solvent effects on the fluorescence of 1-(dimethylamino)-5-naphthalenesulfonic acid and related compounds. J Am Chem Soc 97:3118–3126
Prodi L, Bolletta F, Montalti M, Zaccheroni N (2000) Luminescent chemosensors for transition metal ions. Coord Chem Rev 205:59–83
Talanova GG, Talanov VS (2010) Dansyl-containing fluorogenic calixarenes as optical chemosensors of hazardous metal ions: a mini-review. Supramol Chem 22:838–852
Patra S, Paul P (2009) Synthesis, characterization, electrochemistry and ion-binding studies of ruthenium(II) bipyridine receptor molecules containing calix[4]arene-azacrown as ionophore. Dalton Trans 40:8683–8695
Boricha VP, Patra S, Chouhan YS, Sanavada P, Suresh E, Paul P (2009) Synthesis, characterization, electrochemistry and ion-binding studies of ruthenium(II) and rhenium(I) bipyrydyl crown ether receptor molecules, Eur J Inorg Chem 1256–1267
Maree D, Nyokong T, Suhling K, Phillips D (2002) Effects of axial ligands on the photophysical properties of silicon octaphenoxyphthalocyanine. J Porphyr Phthalocyanine 6:373–376
Fery-Forgues S, Lavabre D (1999) Are fluorescence quantum yields so tricky to measure? A demonstration using familiar stationery products. J Chem Educ 76:1260–1264
El-Sedik M, Almonasy N, Nepras M, Bures F, Dvorak M, Michl M, Cermak J, Hrdina R (2012) Synthesis, absorption and fluorescence properties of N-triazinyl derivatives of 2-aminoanthracene. Dyes Pigments 92:1126–1131
Wurth C, Gonzalez MG, Niessner R, Panne U, Haisch C, Genger UR (2012) Determination of the absolute fluorescence quantum yield of rhodamine 6 G with optical and photoacoustic methods – Providing the basis for fluorescence quantum yield standards. Talanta 90:30–37
Murphy CB, Zhang Y, Troxler T, Ferry V, Martin JJ, Jones WE (2004) Probing Forster and Dexter energy-transfer mechanisms in fluorescent conjugated polymer chemosensors. J Phys Chem B 108:1537–1543
Larsen RW, Helms MK, Everett WR, Jameson DM (1999) Ground- and excited-state characterization of an electrostatic complex between tetrakis(4-sulfanato-phenyl)porphyrin and 16-pyrimidinium crown-4. Photochem Photobiol 69:429–434
Michon J, Frelon S, Garnier C, Coppin F (2010) Determinations of Uranium(VI) Binding Properties with some Metalloproteins (Transferrin, Albumin, Metallothionein and Ferritin) by Fluorescence Quenching. J Fluoresc 20(2):581–590
Acknowledgments
The authors gratefully acknowledge the financial assistance provided by University Grant Commission (UGC), New Delhi. The authors also acknowledge CDRI (Lucknow), GFSU (Gandhinagar) and CSMCRI (Bhavanagar) for providing instrumental facilities and INFLIBNET, Ahmedabad, for e-journals.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Fig. 7. Quenching Comparison of metal ions (Fe(III), Co(II) and Cu(II)) with their intensity. Fig. 8. Graph of % quenching of TDCR with various metal ions. Fig. 9 Color changes of TDCR in Methanol (20μM) in the presence of 20 eq. of metal ions. Fig. 10 Graphical abstract. Fig. 11. NMR Spectra of ligand CR and TDCR. Fig. 12 Mass Spectra of ligand CR and TDCR. (PDF 347 kb)
Rights and permissions
About this article
Cite this article
Bhatt, K.D., Gupte, H.S., Makwana, B.A. et al. Calix Receptor Edifice; Scrupulous Turn Off Fluorescent Sensor for Fe(III), Co(II) and Cu(II). J Fluoresc 22, 1493–1500 (2012). https://doi.org/10.1007/s10895-012-1086-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-012-1086-5