Abstract
A simple and sensitive method has been developed and validated for the determination of aliskiren (ALS) in its dosage forms and spiked plasma. The method was based on the reaction of the drug with dansyl chloride in the presence of bicarbonate solution of pH 10.5 to give a highly fluorescent derivative which was measured at 501 nm with excitition at 378 nm in dichloromethane. Different experimental parameters affecting the development of the method and stability were carefully studied and optimized. The calibration curves were linear over the concentration ranges of 100–700 and 50–150 ng/mL for standard solution and plasma, respectively. The limits of detection were 27.52 ng/mL in standard solution, 4.91 ng/mL in plasma. The developed method was successfully applied to the analysis the drug in the commercial tablets and spiked plasma samples. The mean recovery of ALS from tablets and plasma was 100.10 and 97.81%, respectively. A proposal of the reaction pathway was presented.
Similar content being viewed by others
Introduction
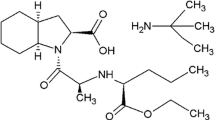
Aliskiren hemifumarate [(2S,4S,5S,7S)-N-(2-carbamoyl-2-methyl propyl)-5-amino-4-hydroxy-2,7-diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy)phenyl]-octanamide hemifumarate] (ALS) is a direct renin enzym inhibitor and recently has been approved for the treatment of essential hypertension [1–3].
There is no official analytical method for analysis of ALS. Literature survey revealed that a some analytical methods (mostly for investigation of pharmacokinetics properties of drug) for determination of ALS in serum and plasma have been reported. These methods include radioimmunoassay [4], heigh performance liquid chromatography (HPLC) [5], heigh performance liquid chromatography/mass spectrometry (HPLC/MS) [6–9], HPLC/MS/MS [1–3] detection. There are only two HPLC [10, 11] and a spectrophotometric methods [10] for the determination of the drug in pharmaceutical dosages. The HPLC coupled with mass spectrometry methods offered the required sensitivity for the analysis of ALS in biological fluids; however their sophisticated instrumentation and high analysis cost limited their use for analysis of drugs. The reported spectrophotometric and HPLC medhods for estimation of ALS in tablets [10] are not sensitive enough with the initial determined concentration of analyte (40 and 25 μg/mL, respectively) and the method may not also apply to analysis of drug in biological fluids. Moreover, the validation results were not given in the reported HPLC method [10, 11]. Although, spectrofluorimetry is a sensitive, selective and simple technique, however up to now, no method has been reported for the determination of ALS in tablets and human plasma by spectrofluorimetry. For these reasons, and novelty of the drug, the development of new sensitive and simple spectrofluorimetric method is very essential. DNS-Cl is known to react with primary, secondary amines, imidazoles, thiols, phenolic and alcoholic hydroxyl groups and carboxylic acid groups [12–14]. DNS-Cl has been used as a fluorogenic reagent for the determination of many pharmaceutical compounds [15–17].
The present study describes a simple, sensitive, fast and cheap spectrofluorometric method for the analysis of ALS in its tablets and spiked plasma samples. The developed method was based on its reaction with dansyl chloride (DNS-Cl) in bicarbonate solution of pH 10.50, to form fluorescent product which was measured fluorometrically at 501 nm (λex 378 nm).
Experimental
Apparatus
Shimadzu RF-1501 (Tokyo, Japan) spectrofluorometer and Shimadzu UV-160 (Tokyo, Japan) UV–vis spectrophotometer were used. One centimeter path length quartz cells were used. pH measurements were made with WTWpH 526 digital pH Meter.
Materials and Reagents
All reagents and solvents were of analytical reagent grade. ALS was kindly supplied by Novartis Pharmaceutical (Istanbul-Turkey) and its film-coated Tekturna® tablets containing 150 mg and 300 mg of ALS were obtained from local pharmacy in Chicago-USA. 5- dimethylaminonaphthalene-1-sulphonyl chloride (DNS-Cl) purchased from Sigma-Aldrich (Germany). Solution of DNS-Cl was freshly prepared at 2.0 mg/ml in acetonitrile.
The sodium bicarbonate (0.025 M) solution was prepared in water and adjusted pH to 10.5 with 0.1 M sodium hydroxide using a pH meter. This solution was kept in refrigerator and used for about 1 week.
Water was purified by aquaMAXTM-ultra, Young-lin instrument (Korea) ultrawater purification system.
Solutions
A stock solution at 1.0 mg/mL (concentration expressed as base compound) was prepared in water. This stock solution was further diluted with water to obtain standard solutions at 0.01 mg/mL. The solutions were stable for at least one month when kept in the refrigerator.
General Procedures
Procedure for Calibration Curve
Aliquots of 50.0–350.0 μL of standard drug solutions (0.01 mg/mL) were transferred into a series of 10 mL stoppered glass tubes. A 300 μL of bicarbonate solution at pH 10.5 and 300 μL of DNS-Cl solution were added to each tube and the mixture was left for 20 min at 30 °C in the dark. Then, the derivative was extracted with 5 mL of dichloromethane by a vortex mixer. The organic layer was separated and dried over anhydrous sodium sulfate. The fluorescence intensity of the resulting solution was measured at 501 nm after excitation at 378 nm. Blank experiment was carried out simultaneously. The corrected fluorescence intensity was plotted versus the final drug concentrations to get the calibration curve. The corresponding regression equation was derived.
Procedure for Spiked Human Plasma
Drug free human plasma was obtained from Istanbul University Cerrahpasa Hospital (Istanbul, Turkey) and stored at −20 °C until analysis and thawed to room temperature before use. A 0.5 mL aliquot of human plasma was transferred into a series of centrifuge tubes and spiked with different concentration of ALS to give a final concentration range of 50–150 ng/mL (Table 1). Extraction with 1.0 mL acetonitrile was performed to precipitate plasma proteins, followed by centrifugation at 4,500 rpm for 20 min. The resulting supernatant was evaporated to dryness under nitrogen at 40 °C. The residual mass was reconstituted with 0.1 mL water and then the analyses were performed as under “Procedure for Calibration Curve”.
All the procedures for blank were performed in the same manner. The corrected fluorescence intensity was measured at excitation wavelength of 378 nm and emission wavelength of 501 nm respectively.
Procedure for Tablet Formulations
Ten Tekturna tablets containing 150 and 300 mg ALS were weighed and powdered separately. From each of the powdered tablet forms, an amount of the powder equivalent to 150 and 300 mg ALS were transferred to 100 mL volumetric flasks seperately. About 50 mL of water was added and then extraction was performed mechanically for 1 h. The volumes were brought to 100 mL with water and final solution was filtered. For both tablet solution, aliquot covering the working concentration range (cited in Table 1) was trasferred into glass tubes and analyzed separately as described under “Procedure for Calibration Curve” section.
The nominal content of the tablets was determined using the corresponding regression equations of the calibration curves.
Determination of the Stoichiometric Ratio of the Reaction
The reaction stoichiometry between ALS and DNS-Cl was determined by Job’s continuous variation method. The drug solution with identical molar concentrations of reagent (2.5 × 10−4 M) were mixed in varying volume ratios ((5:1, 4.8:1.2, …, 1.2:4.8, 1:5) in which the total volume of the mixtures were kept at 6.0 mL, and the procedures were completed as described for the calibration curve. The fluorescence intensity values obtained were plotted against the mole fraction of the drug solution. Each determination was carried out in triplicate.
Results and Discussion
Optimization of Experimental Parameters
The different experimental parameters affecting the development of the reaction product and its stability were studied and optimized for the spectrofluorimetric method. Such factors were changed individually while others were kept constant. These factors include pH, type of buffer, amounts of reagent, temperature, reaction time and effect of solvent.
Effect of pH
The influence of pH on the fluorescence intensity of the reaction product was examined using bicarbonate solution and borate buffer over the pH range from 9.2 to 11.0 since DNS-Cl reacts under alkaline conditions. The maximum fluorescence intensity was obtained when the reaction was carried out with bicarbonate solution of pH 10.5 (Fig. 1) and the optimum volume of this solution was 0.3 mL.
Effect of DNS-Cl Concentration
The effect of the concentration of DNS-Cl was studied using different volumes of the reagent at 2 mg/mL solution (varied from 50 mL to 350 μL). It was found that increasing the volume of the reagent produces a proportional increase in the fluorescence intensity of the reaction product up to 300 μL. However, no further increase in the fluorescence intensity was observed upon increasing the volume of the reagent up to 350 μL. Therefore, 300 μL of 2 mg/mL of DNS-Cl solution was chosen for derivatization reaction.
Effect of Reaction and Heating Time
Different reaction times (5–30 min) and temperatures (at room and 30–60 °C) were studied to obtain highest fluorescence intensity of the reaction product. It was found that the reaction product reached the highest fluorescence within 20 min at 30 °C. The effect of temperature on the reaction of ALS with DNS-Cl was shown in Fig. 2.
Effect of Extraction Solvent
In order to avoid the interference of the highly fluorescent dansyl hydroxide formed in the dansylation reaction medium, which is polar character and remains in the aqueous phase, the derivatization product was extracted with immiscible organic solvent for spectrofluorimetric method. For this purpose, different solvents including tetrachloromethane, diethyl ether, dichloromethane and chloroform were tested and the highest fluorescence was obtained upon using dichloromethane (Table 1). The emission and excitation spectrum of derivatization product of ALS in dichloromethane are shown in Figs. 3 and 4. The fluorescence intensity of the derivatization product in the same solvent was stable for at least 24 h if stored in dark at room temperature.
Stoichiometry and Mechanism of the Reaction
Under the selected conditions, the stoichiometry of the reaction between ALS and DNS-Cl was studied by Job’s method of continuous variation [18]. As shown in Fig. 5, the stoichiometry of the reaction was found as 1:2 ratios (drug/reagent), confirming that one molecule of ALS reacts with two molecule of DNS-Cl.
ALS contains a primary aliphatic amine and secondary hydroxyl group which are capable of acting as base and involved nucleophilic reactions. Thus, the sulfonyl chloride group of DNS-Cl can be attacked by these nucleophilic groups. Also, the acidity of these groups plays an important factor in the dansylation reaction. Comparison of acidity based on pKa appears as follows: R-NH2 (pKa~36), ROH (pKa~16), RNHR (pKa~11) [13]. Based on all these fact and by the previous dansylation studies, it is concluded that ALS in alkaline medium (pH = 10.5) reacts with DNS-Cl through its primary amino group and hydroxyl group. In order to confirm the placement of dansyl moiety on drug, IR spectra (Perkin–Elmer Universal ATR sampling accessory apparatus) of ALS (Fig. 6) and derivatization product (Fig. 7) which was prepared as the same manner of described in experimental section were also recorded and compared. IR spectrum did not provide more information since several absorption bands for the stretching of O-H and N-H bonds were observed in the same regions. However, the extinction of characteristic O-H signal at 1,284 cm−1 [19] in the IR spectrum of the reaction product provided that one DNS moiety was attached to the drug molecule at hydroxyl group.
A schematic proposal of the reaction pathway is given in Scheme 1.
Analytical Performance
Linearity and Sensitivity
For evaluation of linearity at the selected conditions, determination of ALS was carried out at six concentration (n = 6) and five concentration levels for standard solution and plasma samples (n = 5), respectively. The calibration curves of ALS were linear over the concentration range of 100–700 ng/mL for standard solution and 50–150 ng/mL for plasma samples.
The limit of detection (LOD) and limit of quantification (LOQ) were calculated as 3S b /m and 10S b /m, respectively, S b is the standard deviation of the intercept of regression line, and m is the slope of the calibratiom curve [20]. On this basis, the LOD and LOQ of the proposed method for standard drug analysis were 27.53 and 91.76 ng/mL for ALS, respectively. The LOD and LOQ for plasma samples were 4.91 and 16.37, respectively. The parameters for the analytical performance of the proposed method are summarized in Table 2.
Accuracy and Precision
Accuracy, intraday and interday precisions of the method were determined. Five replicate spiked plasma samples in the same day, as well as on five consecutive days were assayed for intra-day and inter-day accuracy at three different concentrations for each analyte. Accuracy was calculated as deviation of the mean from the nominal concentration. The intraday and interday precisions (expressed as the relative standard deviation (RSD%) for ALS ranged from 0.47 to 2.51% and 0.58 to 2.19%, respectively. The intraday and interday precisions for drug in plasma ranged from 0.37 to 2.09% and 0.96 to 2.30%, respectively.
Recovery
Recovery studies were carried, by spiking known different amounts of pure drug solutions to the preanalysed drug samples. The results given in Table 3 revealed that the RSD% and percent average of recovery for preparation samples were in the range of 0.99–2.73%, 99.75–101.37%. Recovery results suggest method to be unaffected in the presence of formulation excipients and confirm the heigh accuracy.
The RSD% and percent average of extraction recovery for ALS determined at different concentrations in plasma were in the range of 0.55–2.14%, 96.08–98.75%, respectively (Table 3).
Robustness
Robustness was examined by evaluating the influence of small variations in the experimental conditions such as working excitation and emission wavelengths (±3 nm), volume of reagent (±5 μL), volume of bicarbonate solution (±10 μL), change in pH (±0.2), the temperature degree and heat time, which are applied for derivatization reaction (±5 °C and ±5 min). These minor changes that may take place during the experimental operation did not have any significant effect on fluorescence intensity of the reaction product. RSD% for the measured fluorescence intensity after the studied variations did not exceed 4.50%.
Application to Tablets
The proposed method was successfully applied to analysis of two different commercial tablets (Tekturna Tablets) which contain 150 and 300 mg ALS. The mean recovery values of tablets that were contained 150 mg and 300 mg ALS were 100.18% and 100.03%, respectively (Table 4).
Conclusion
The proposed spectrofluorimetric method has the advantage of being simple, fast, highly sensitive, accurate, low cost, and do not require any pretreatment of the drug and tedious extraction procedure. The method was successfuly applied for determining ALS in tablets and spiked plasma without any interference from excipients. Therefore, the developed method can be suitable for routine analysis of ALS in quality control laboratories and clinical laboratories.
References
Huang HL, Vaidyanathan S, Yeh CM, Bizot MN, Dieterich HA, Dole WP, Howard D. Effect of aliskiren, an oral direct renin inhibitor, on the pharmacokinetics and pharmacodynamics of a single dose of acenocoumarol in healthy volunteers. Curr Med Res Opin 24:2449–2456
Limoges D, Dieterich HA, Yeh CM, Vaidyanathan S, Howard D, Dole WP (2008) A study of dose-proportionality in the pharmacokinetics of the oral direct renin inhibitor aliskiren in healthy subjects. Int J Clin Pharmacol Ther 46:252–258
Zhao C, Vaidyanathan S, Yeh CM, Maboudian M, Armin Dieterich H (2006) Aliskiren exhibits similar pharmacokinetics in healthy volunteers and patients with type 2 diabetes mellitus. Clin Pharmacokinet 45:1125–1134
Lefévre G, Duval M, Poncin A (2000) Direct micro-radioimmunoassay of the new rennin inhibitor CGP 60536. J Immunoassay 21:65–84
Lefevre G, Gauron S (2000) Automated quantitative determination of the new rennin inhibitor CGP 60536 by high-performance liquid chromatography. J Chromatogr B 738:129–136
Dieterle W, Corynen S, Vaidyanathan S, Mann J (2005) Pharmacokinetic interactions of the oral renin inhibitor aliskiren with lovastatin, atenolol, celecoxib and cimetidine. Int J Clin Pharmacol Ther 43:527–535
Vaidyanathan S, Valencia J, Kemp C, Zhao C, Yeh CM, Bizot MN, Denouel J, Dieterich HA, Dole WP (2006) Lack of pharmacokinetic interactions of aliskiren, a novel direct rennin inhibitor for the treatment of hypertension, with the antihypertensives amlodipine, valsartan, hydrochlorothiazide (HCTZ) and ramipril in healthy volunteers. Int J Clin Pract 60:1343–1356
Ayalasomayajula S, Tchaloyan S, Yeh CM, Bizot MN, Dieterich HA, Howard D, Dole WP (2008) A study of the pharmacokinetic interactions of the direct renin inhibitor aliskiren with allopurinol, celecoxib and cimetidine in healthy subjects. Curr Med Res Opin 224:717–726
Vaidyanathan S, Bigler H, Yeh C, Bizot MN, Dieterich HA, Howard D, Dole WP (2007) Pharmacokinetics of the oral direct renin inhibitor aliskiren alone and in combination with irbesartan in renal impairment. Clin Pharmacokinet 46:661–675
Wrasse-Sangoi M, Secretti LT, Diefenbach IF, Rolim CMB, Sangoi MD (2010) Development and validation of an UV spectrophotometric method for the determination of aliskiren in tablets. Quim Nova 33:1330–1334
Pachauri S, Paliwal S, Srinivas KS, Singh Y, Jain V (2010) Development and validation of HPLC method for analysis of some antihypertensive agents in their pharmaceutical dosage forms. J Pharm Sci Res 2:459–464
Ayad MM, el-Hay MH (1984) Spectrofluorimetric micro-determination of imidazoline derivatives using 1-dimethylaminonaphthalene-5-sulphonyl chloride. Analyst 109:1431–1434
Bartzatt R (2003) Dansylation of aromatic, aliphatic and medicinal carboxylic acid compounds in 1M Na2CO3 buffer. Anal Chim Acta 488:203–209
Ma Y, Xiang F, Jin W, Yu L (2010) Determination of total glutathione in yeasts by highperformance liquid chromatography with dansylation. Z Naturforsch C 65:391–394
El-Enany N, Belal F, Rizk M (2008) Spectrofluorimetric determination of oxamniquine in dosage forms and spiked human plasma through derivatization with 1 dimethylaminonaphthalene-5-sulphonyl chloride. J Fluoresc 18:349–355
Zhuang XM, Yuan M, Zhang ZW, Wang XY, Zhang ZQ, Ruan JX (2008) Determination of 4-dimethylaminophenol concentrations in dog blood using LC-ESI/MS/MS combined with precolumn derivatization. J Chromatogr B Analyt Technol Biomed Life Sci 876:76–82
Cruces-Blanco C, Segura Carretero A, Merino Boyle E, Fernández GA (1999) The use of dansyl chloride in the spectrofluorimetric determination of the synthetic antioxidant butylated hydroxyanisole in foodstuffs. Talanta 50:1099–1108
Job P (1928) Formation and stability of inorganic complexes in solution. Anal Chem 9:113–203
Coates, J. (2000). Encyclopedia of analytical chemistry: interpretation of infrared spectra, a practical approach. R.A. Meyers (Ed.), John Wiley and Sons Ltd, Chichester. pp. 10815–10837
Validation of Analytical Procedures. Methodology ICH harmonised tripartite guideline having reached step 4 of the ICH process at the ICH Steering Committee Meeting on, 1996, November 6
Acknowledgement
This work was supported by Research Fund of the Istanbul University. Project number: BAP-4045.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Aydoğmuş, Z., Sarı, F. & Ulu, S.T. Spectrofluorimetric Determination of Aliskiren in Tablets and Spiked Human Plasma through Derivatization with Dansyl Chloride. J Fluoresc 22, 549–556 (2012). https://doi.org/10.1007/s10895-011-0988-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-011-0988-y