Abstract
Using Pb2+ as ion perturber, phenosafranine (PF) and fluorescein isothiocyanate (FITC) could emit strong and stable room temperature phosphorescence (RTP) signal on the filter paper, respectively. When they were mixed, the phenomenon that the RTP signal of PF and FITC enhanced significantly was found. And 1.12 ag DNA spot−1 (sample volume was 0.40 μL, corresponding concentration was 2.8 × 10–15 g mL–1) could cause the RTP signal of both PF and FITC to enhance sharply. The content of DNA was proportional to the ΔI p of PF and FITC in the system at 634 and 659 nm. Thus, a new solid substrate room temperature phosphorimetry (SSRTP) for the determination of trace DNA was established by using FITC-PF as double-luminescent phosphorescence probe. The detection limit (LD) of this method calculated by 3Sb/k was 14 zg DNA spot–1 for PF and 18 zg DNA spot–1 for FITC, respectively, showing high sensitivity. It has been applied to the determination of trace DNA in practical samples and the analysis results were in accordance with those of fluorescence probe. The reaction mechanism of SSRTP for the determination of trace DNA was also discussed.
Similar content being viewed by others
Introduction
DNA is not only the basic genetic material of all living beings, but also the main component of chromosomes [1], and it has close relationship with life activities and all kinds of metabolism [2]. Obviously, the determination of DNA content has an important significance [3]. With the development of science and technology, many new methods for the determination of DNA content emerged [4], such as spectrophotometry (LD: 4.0 × 10–9 g mL–1) [5], fluorimetry (LD: 2.7 × 10–8 g mL–1) [6], near-infrared fluorescence probe method (LD: 6.4 × 10–8 g mL–1) [7], resonance light scattering spectroscopy (LD: 1.9 × 10–8 g mL–1) [8], CdTe / CdS core-shell quantum dot fluorescent probe (LD: 2.0 × 10–8 g mL–1) [9], CdS fluorescence probe method (LD: 1.8 × 10−8 g mL–1) [10], Morin·SiO2 phosphorescent probe method (LD: 1.5 × 10−12 g mL−1) [11], polymerase chain reaction (PCR) technique [12] and so on. Recently, phosphorescent probes have become efficient tools which can explore the nature of micro-environment or biological macromolecules in organic medium (like configurational changes of nucleic acid and protein) and the reaction mechanism between them and drugs [13]. Phosphorescence has higher sensitivity, and biological molecules have no RTP emission in the near-infrared long-wave region. Therefore, the quest and synthesis of a probe which can emit RTP in the long-wave region, to eliminate self-interference of biological molecules and to promote selectivity of RTP probe, has special significance to the study of biological molecules, especially, of the concentration and configurational changes of DNA. At the same time, it has a mutual complementation and verification with nucleic acid fluorescent probe [14]. In this paper, we developed a new double-luminescent phosphorescence probe which could emit strong and stable RTP in the long-wave region to further enhance the sensitivity, selectivity and flexibility of SSRTP for the determination of ultra-trace DNA and the research of life science.
In this experiment, we found that FITC-PF could interact with DNA, which resulted in the dramatical enhancement of RTP signal on filter solid substrate, and showed the superior characteristics of a double-luminescent phosphorescence probe. The optimal conditions, analytical parameters, selectivity and the application of SSRTP for the determination of DNA were discussed using FITC-PF as a double-luminescent phosphorescence probe. Compared with a single-luminescent phosphorescence probe [11], the RTP signal of FITC or PF in FITC-PF double-luminescent phosphorescence probe could be used to determine DNA, which not only enhanced the flexibility of SSRTP, but also provided a sensitive, accurate and reliable analytical method to determine trace DNA in biological samples and promoted life science research.
Experimental
Apparatus and Reagents
A LS-55 luminescence spectrophotometer with a solid surface analysis apparatus (Perkin Elmer Corporation of American, main parameters are: Ex. Slit: 10 nm; Em. Slit: 10 nm; scan speed: 1,500 nm min–1), an AE240 electronic analytical balance (Mettler-Toledo Instruments Company Limited), infrared spectrophotometer (Necolet-360 Nicolet Company, USA) and a 0.50-μL (± 0.010 μL) flat head micro-injector (Shanghai Medical Laser Instrument Plant) were used.
CT-DNA working solution (Sigma Company): 1.0 mg mL–1 stock solution was prepared with water and placed at 4°C in the refrigerator for use; working solution: diluted 1.0 mg mL–1 stock solution to 10.00 and 1.00 (fg mL–1) gradually with water; 1.0 × 10–4 mol L–1 PF solution; 1.0 × 10–4 mol L–1 FITC solution. All reagents were A.R. grade except that DNA was biological reagent. The water used was thrice-distilled water.
Filter paper, PAM, acetic cellulose membrane (ACM) and nitric cellulose membrane (NCM) were purchased from Luqiaosijia Biochemical Plastic Plant. They were cut into discs (Ф = 15 mm) and a ring indentation (Φ = 4.0 mm) was made at the center of each sheet with a standard pinhole plotter for use.
Experimental Method
To a 25-mL colorimetric tube, a certain amount of DNA, 1.50 mL of 4.0 × 10−5 mol L–1 FITC and 0.15 mL of 5.0 × 10−4 mol L–1 PF were added and diluted with water, and finally mixed homogeneously. The tube was kept at 40°C for 15 min, and then cooled by flowing water for 5 min. 0.40 μL of different concentrations of DNA and blank solution were suspended onto the center of the paper by a 0.50-μL micro-injection, and then dried at 90 ± 1°C for 2 min. Then it was immersed in 1.0 mol L–1 Pb(Ac)2 solution for 10 s, and dried at 90 ± 1°C for 2 min. The phosphorescence intensity of test solution (Ip1) and reagent blank (Ip0) were directly measured at λ maxem = 633.5 nm (PF) or λ maxem = 659.0 nm (FITC). Then ΔIp (= Ip1–Ip0) was calculated.
Infrared Spectra Analysis of FITC, PF and FITC-PF
To a 25-mL colorimetric tube, 15.0 mL of 4.0 × 10−5 mol L–1 FITC and 1.5 mL of 5.0 × 10−4 mol L–1 PF were added and diluted with water, and finally mixed homogeneously. The tube was kept at 40°C for 15 min, and then cooled by flowing water for 5 min. FITC-PF (salmon pink spot, R f = 0.43) was obtained by layer chromatography (chloroform : methanol : acetic acid = 250: 25 : 1). Infrared spectra of FITC, PF and FITC-PF were scanned after the sample preparation by pressed disc method with KBr.
Results and Discussion
Phosphorescence Spectra of the FITC-PF System
The phosphorescence spectra of the FITC-PF system were scanned by the experimental method (Fig. 1, Table 1). Using Pb2+ as the ion perturber, PF and FITC could emit strong and stable RTP signal on filter paper solid substrate. And their λ maxem were 611.6 nm and 634.5 nm, I p values were 40.1 and 64.8, respectively. However, the RTP signals of PF and the FITC in the FITC-PF system were significantly enhanced, their λ maxem were 621.5 nm and 646.0 nm, I p values were 56.2 and 93.5, respectively, namely their λ maxem red shifted for 9.9 nm and 11.5 nm, which prompted that there was a new compound formed. When 70.0 fg DNA was added, the RTP signal of PF and FITC in FITC-PF system were dramatically increased, their λ maxem were 633.5 nm and 659.0 nm, I p values were 103.2 and 86.5, with the λ maxem red shifting for 12.1 nm and 13.0 nm, respectively. The reason might be that PF and FITC interacted with DNA to generate new materials, respectively. According to the relationship between RTP signal and DNA content, the possibility of SSRTP for the determination of DNA content was revealed using FITC-PF as a double-luminescent phosphorescence probe.
The Optimal Determination Conditions
For the system containing 0.48 ag DNA spot−1, the effects of the concentration and volume of PF, FITC, acidity of reaction, temperature and time of reaction, solid substrate, standing time, the species of ion perturber and concentrations of Pb2+ on the ∆Ip of the system were tested, respectively (Table 2). The results showed that the ∆Ip of the system reached the maximum when 0.15 mL of 3.0 × 10–6 mol L–1 PF、1.50 mL of 2.4 × 10–6 mol L–1 PF were used, the pH of reaction system was 5.42, the reaction temperature was 40°C and the time was 15 min, time of passing N2 was 10 min, paper as solid substrate as well as 1.0 mol L–1 Pb2+ was used.
Working Curve, Linear Range, LD and the Limit of Quantification (LOQ)
Under the optimal determination conditions, the content of DNA ranging from 0.0080 ag spot–1 to 1.12 ag spot–1 (corresponding concentration: 0.020–2.8 fg mL–1, sample volume: 0.40 μL) had good linear relationship with the ΔI p of the system (When the content of DNA were 0, 0.008, 0.16, 0.48, 0.80, and 1.12 ag spot−1, the ΔI p of the system were 0, 4.9, 14.3, 42.9, 71.4, 103.2 (for PF) and 0, 4.4, 11.9, 35.7, 59.5, 86.5 (for FITC), respectively.). When a FITC-PF double-luminescent phosphorescence probe was used to determine DNA content, the regression equations of working curve were ΔI p = 1.600 + 89.06 mDNA (ag spot–1) and ΔI p = 1.391 + 74.39 mDNA (ag spot–1); correlation coefficients (r) were 0.9987 and 0.9984; RSDs were 1.2–4.5% and 1.5%–3.4% (measured 0.020 and 1.12 ag spot–1 DNA for 7 parallel determination to calculate the RSD); LD were 2.8 ag spot–1 and 3.3 ag spot–1 (measured reagent blank for 11 parallel determination, calculated by 3Sb/k and Sb were 0.042 and 0.046, respectively.), LOQ were 9.2 ag spot–1 and 10.9 ag spot–1 (calculated by 10Sb/k), respectively. The results showed that this method was of good precision and high sensitivity.
Interference Experiment
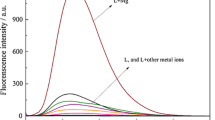
Under the optimal conditions, the DNA content was measured by this method (1.2 fg mL–1) and those in the literatures [14] (1.0 ng mL–1) and [15] (0.10 μg mL–1). When the relative error (Er) was within ± 5%, the maximum allowed concentrations of coexistences are listed in Table 3.
The results showed that the maximum allowed concentrations of coexistences of this method were higher than those of the literatures [10, 11], which indicated high selectivity. And the maximum allowed concentrations of common cations (Mg2+, K+, Ca2+), which might combine with the phosphate group (P) of DNA [15], were higher with less interference. While the maximum allowed concentrations of Mn2+, Cr3+, Fe3+, Co2+ were lower, the reason might be that they combined with the bases of DNA [15] and destroyed the hydrogen bonds, which led to acute changes in DNA structure. For the reason that the allowed concentrations of interference ions were higher than those in biological body, the interference of this method used to determine biological fluids was less. The allowed concentration of yeast RNA was 4.5 mg L–1, so this method was suitable for the determination of RNA.
Sample Analysis
1.0 g (± 0.10 mg) A, B, C, D, E and F nectar were weighed and treated according to the method mentioned in Ref. [16], and diluted to 100 mL with water; 1.00 mL test solution were taken and diluted to 100 mL with water. Taking 1.00 mL test solution and measuring the DNA content of samples according to the experimental method and that in literature [16]. The results are listed in Table 4.
Seen from Table 4, no matter the working wavelength of FITC or that of PF in FITC-PF double-luminescent phosphorescence probe was used to determine the DNA content in honey A, B, C, D, E, F and G, the recovery rates (%) were within 99.0–100.2 and 98.4–100.0, RSDs (%) were in the context of 3.5–4.5 and 3.2–4.3, respectively, which showed that this method was of good flexibility, high accuracy and precision.
Reaction Mechanism
Using Pb2+ as perturber, both PF and FITC on filter paper solid substrate could emit strong and stable RTP signal after reacting at 40°C for 15 min; when they were mixed, the RTP signal of PF and FITC significantly enhanced with the λ maxem red shifting for 9.9 nm and 11.5 nm, the reason might be that the -NCS [17] in FITC molecules reacted with the -NH2 in PF molecules to produce new compounds FITC-PF (Fig. 2) which contained −NH−CS−NH− bond:
The infrared spectra of FITC, PF and FITC-PF was scanned in order to discussed the possibility that FITC reacted with PF to form FITC-PF. And the results are listed in Table 5.
The infrared spectra of FITC-PF kept the most characteristic adsorption peak of FITC and PF, but the stretching vibration peak of −NCS (ν: 2,050 cm–1) in the range of 2,150–2,020 cm–1 disappeared and appeared a new stretching vibration peak of −N−CS−N− (ν: 1,380 cm–1) in the range of 1,430–1,130 cm–1. Thus, it could conclude that the −NCS of FITC reacted with −NH2 to form the FITC-PF.
When 70.0 fg DNA existed, the RTP signal of PF and FITC in FITC-PF system dramatically increased with the λ maxem red shifting for 12.1 nm and 13.0 nm, respectively. The reason might be the -COOH in H2N-Pr-COOH (DNA) molecule reacted with the -NH2 in FITC-PF to produce FITC-PF-DNA (Fig. 3).
According to the linear relationship between DNA content and the ΔI p of the system, SSRTP can be used to determine DNA content using FITC-PF as a double-luminescent phosphorescence probe.
Conclusion
In this paper, FITC-PF double-luminescent phosphorescence probe was developed, a new SSRTP for the determination of trace DNA was estabilished, DNA content in honey was determined by the emission wavelength of FITC and PF in double-luminescent phosphorescence RTP probe, respectively. The flexibility and applicability of SSRTP have been improved, showing broader application prospect. If FITC-PF double-luminescent phosphorescence probe is used to label antibodies and lectins, SSRTP immunization method or SSRTP affinity adsorption method for the determination of trace biological active substances (such as IgG, IgA, IgM, IgE, alkaline phosphatase, alpha-fetoprotein heterogeneity and so on.) will be established, showing the application prospect of phosphorescence probe.
References
Li Z, Lai JP, Wu CL, Guo XQ, Zhao YY (2006) Study on the interaction mechanism of DNA and Tb3+ by fluorimetry. J Xiamen Univ (Nat Sci) 45(1):76–79
Luan JM, Zhang XD (2006) Progress of the study on the mode of combination of bioprobes with DNA and of the determination of DNA by photometry. PTCA Part B: Chem Anal 42:964–968
Topal MD, Fresco JR (1980) Fluorescence of terbiumion-nucleic acid complexes: a sensitive specific probe for unpaired residues in nucleic acids. Biochem 19(24):5531–5537
Ding C, Cantor CR (2004) Quantitative analysis of nucleic acids the last few years of progress. J Biochem Mol Biol 37(1):1210
Wei XP, Sun XY (2006) Kinetics spectrophotometric determination of nucleic acid. J Guilin Univ Technol 26(4):562–564
Guo LQ, Ye FG, Lin XC, Xie ZH (2006) Determination of nucleic acids using toluidine blue as a fluorescence probe. Spectrosc Spect Anal 26(1):121–124
Hu M, Zhang ZX, Shen GL, Liu YL (2007) Near-infrared fluoresent probe of azure a for the determination of deoxyribonucleic acid. Chin J Anal Chem 35(6):890–892
Feng S, Li ZP, Wu QH, Fang Z, Zhang SH (2007) Resonance scattering spectrometric determination of DNA with polyamine as a probe. PTCA (Part B: Chem Anal) 43(7):555–557
Xu J, Zhao YS, Wang HM, Wu XG, Cai RX (2006) Application of CdTe/CdS core-shell quantum dots synthesized in an aqueous solution as fluorescence probe for the determination of nucleic acids. Chin J Anal Lab 25(4):50–53
Wang L, Guo C, Li MG, Xu FG, Zhu CQ, Wang L (2003) Fluorescence quenching method for the determination of nucleic acids with functionalized CdS nanoparticles as fluorescence probes. Chin J Anal Chem (31):83-86
Liu JM, Yang TL, Gao F et al (2006) Determination of DNA by solid substrate room temperature phosphorescence enhancing method based on the morin·SiO2 luminescent nanoparticles–Pd system as a phosphorescence probe. Anal Chim Acta 561:191–197
Chen CF, Ridzon DA, Broomer AJ (2005) Real-time quantification of micro RNAs by stem-loop RT-PCR. Nucleic Acids Res 33(20):179
Yuan W, Jin WJ, Wei YS et al (1999) Chem J Chin Univ 20:344
Feng Q, Li NQ, Jiang YY (1997) Anal Chim Acta 344:97–104
Yang P (1991) Introduction of bioinorganic chemistry. Xi’an Communication University Press, pp 275
Zhu ZC, Wang J, Zhu HB (2002) Determination of nucleic acids using morin-niobium complex as a fluorescence probe. Chin J Anal Chem 30(11):1319–1321
Liu JM, Liu ZB, He HX et al (2008) Determination of glucose by affinity adsorption solid substrate room temperature phosphorimetry based on triticum vulgare lectin labeled with silicon dioxide nanoparticle containing fluorescein isothiocyanate. Chin J Chem 26(9):1635–1640
Acknowledgements
This work was supported by Fujian Province Science Foundation (Grant No. 2010J01053, 2009J1017 and 2008J0313) and Fujian Education Office Science and Technology Item Programme (JA08252, JB08262 and JB09278) and Scientific Research Program of Zhangzhou Institute of Technology Foundation (Grant No. ZZY0942, ZZY0952). At the same time, we are very grateful to precious advices raised by the reviewers and the editorial of Journal of Fluorescence.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Liu, JM., Huang, XM., Liu, ZB. et al. Determination of Trace Deoxyribonucleic Acid by Using Fluorescein Isothiocyanate-Phenosafranine as a Double-Luminescent Phosphorescence Probe. J Fluoresc 21, 195–202 (2011). https://doi.org/10.1007/s10895-010-0705-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-010-0705-2