Abstract
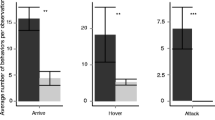
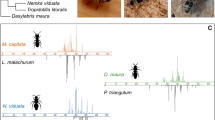
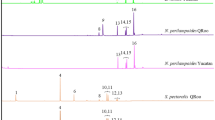
Astigmatid mites in the family Canestriniidae are often closely associated with tortoise leaf beetles (Chrysomelidae: Cassidinae). For example, the survival of the commensal canestriniid mite Grandiella rugosita depends on dispersal to the cassidine beetle Acromis sparsa. Here, we tested whether the beetle cuticle provides chemical cues for host recognition for G. rugosita. In two-choice assays with cuticular extracts from A. sparsa and the co-occurring, non-host cassidine Chelymorpha alternans offered simultaneously, mites clearly preferred the area treated with extract from their host. In no-choice assays, G. rugosita spent three times longer and moved three times slower on host cuticular extracts compared to non-host extracts and the solvent control. Analyses of the chemical composition of cuticular extracts from males and females of A. sparsa and C. alternans revealed complex mixtures of mainly methyl branched hydrocarbons, which clearly separated both species in a principal component analysis. We found no qualitative difference between males and females of either species, but in C. alternans quantitative differences between males and females were detected. Our results demonstrate that G. rugosita is able to discriminate between cuticular extracts from its host A. sparsa and the non-host C. alternans. The components eliciting the observed arrestment behavior remain to be determined.


Similar content being viewed by others
References
Abbot P, Dill LM (2001) Sexually transmitted parasites and sexual selection in the milkweed leaf beetle, Labidomera clivicollis Oikos 92:91–100
Aitchison J (1986) The statistical analysis of compositional data. Chapman & Hall, London
Aumeier P, Rosenkranz P, Francke W (2002) Cuticular volatiles, attractivity of worker larvae and invasion of brood cells by Varroa mites. A comparison of Africanized and European honey bees Chemoecology 12:65–75
Carlson DA, Bernier UR, Sutton BD (1998) Elution patterns from capillary GC for methyl-branched alkanes. J Chem Ecol 24:1845–1865
Del Piccolo F, Nazzi F, Della Vedova G, Milani N (2010) Selection of Apis mellifera workers by the parastic mite Varroa destructor using host cuticular hydrocarbons. Parasitology 137:967–973
Donzé G, Schnyder-Candrian S, Bogdanov S, Diehl PA, Guerin PM, Kilchenman V, Monachon F (1998) Aliphatic alcohols and aldehydes of the honey bee cocoon induce arrestment behavior in Varroa jacobsoni (Acari: Mesostigmata), an ectoparasite of Apis mellifera. Arch Insect Biochem 37:129–145
Dubis E, Malinski E, Dubis A, Szafranek J, Nawrot J, Poplawski J, Wrobel JT (1987) Sex-dependent composition of cuticular hydrocarbons of the Colorado beetle, Leptinotarsa decemlineata Say. Comp Biochem Phys A 87:839–843
Geiselhardt S, Otte T, Hilker M (2012) Looking for a similar partner: host plants shape mating preferences of herbivorous insects by altering their contact pheromones. Ecol Lett 15:971–977
Geiselhardt SF, Geiselhardt S, Peschke K (2011) Congruence of epicuticular hydrocarbons and tarsal secretions as a principle in beetles. Chemoecology 21:181–186
Golden KL, Meinke LJ, Stanley-Samuelson DW (1992) Cuticular hydrocarbon discrimination of Diabrotica (Coleoptera, Chrysomelidae) sibling species. Ann Entomol Soc Am 85:561–570
Horn JL (1965) A rationale and test for the number of factors in factor analysis. Psychometrika 30:179–185
Jacob J, Hanssen HP (1986) Distribution and variability of cuticular hydrocarbons within the Coleoptera. Biochem Syst Ecol 14:207–210
Kováts ES (1965) Gas chromatographic characterization of organic substances in the retention index system. Advances in chromatography 1:229–247
McMullan JB, D'ettorre P, Brown MJF (2010) Chemical cues in the host-seeking behaviour of tracheal mites (Acarapis woodi) in honey bees (Apis mellifera mellifera). Apidologie 41:568–578
Nelson DR, Adams TS, Fatland CL (2003) Hydrocarbons in the surface wax of eggs and adults of the Colorado potato beetle, Leptinotarsa decemlineata. Comp Biochem Phys B 134:447–466
Nelson DR, Charlet LD (2003) Cuticular hydrocarbons of the sunflower beetle, Zygogramma exclamationis. Comp Biochem Phys B 135:273–284
Nelson DR, Sukkestad DR (1970) Normal and branched aliphatic hydrocarbons from eggs of tobacco hornworm. Biochemistry-Us 9:4601–4611
Nelson DR, Sukkestad DR, Zaylskie RG (1972) Mass spectra of methyl-branched hydrocarbons from eggs of tobacco hornworm. J Lipid Res 13:413–421
Nikolova N, Rezanka T, Nikolova-Damyanova B, Kalushkov P (1999) Hydrocarbons in adult Chrysomela vigintipunctata (Scopoli) (Coleoptera : Chrysomelidae). Comp Biochem Phys B 123:67–77
O'Connor BM (1994) Life-history modifications ins astigmatid mites. In: Houck A (ed) M. Mites. Ecological and evolutionary analyses of life-history patterns, Chapman & Hall, New York, pp 136–159
Phelan PL, Smith AW, Needham GR (1991) Mediation of host selection by cuticular hydrocarbons in the honeybee tracheal mite Acarapis woodi (Rennie). J Chem Ecol 17:463–473
Pomonis JG, Nelson DR, Fatland CL (1980) Insect Hydrocarbons. 2. Mass spectra of dimethylalkanes and the effect of the number of methylene units between methyl groups on fragmentation. J Chem Ecol 6:965–972
Rickli M, Diehl PA, Guerin PM (1994) Cuticle alkanes of honeybee larvae mediate arrestment of bee parasite Varroa jacobsoni. J Chem Ecol 20:2437–2453
Sabelis MW, Vermaat JE, Groeneveld A (1984) Arrestment responses of the predatory mite, Phytoseiulus persimilis, to steep odor gradients of a kairomone. Physiol Entomol 9:437–446
Santiago-Blay JA, Fain A (1994) Phoretic and ectoparasitic mites (Acari) of the Chrysomelidae. In: Petitpierre E (ed) P. Jolivet & Cox M.L. Kluwer Cacademic Publishers, Netherlands, Novel aspects of the biology of Chrysomelidae, pp 407–417
Seeman OD, Nahrung HF (2004) Female biased parasitism and the importance of host generation overlap in a sexually transmitted parasite of beetles. J Parasitol 90:114–118
Soroker V, Nelson DR, Bahar O, Reneh S, Yablonski S, Palevsky E (2003) Whitefly wax as a cue for phoresy in the broad mite, Polyphagotarsonemus latus (Acari: Tarsonemidae). Chemoecology 13:163–168
Summers FB, Schuster RO (1979) Eleven new species of Grandiella Lombardini (Acarina: Canestriniidae). Int J Acarol 5:325–350
Waage JK (1978) Arrestment responses of parasitoid, Nemeritis canescens, to a contact chemical produced by its host, Plodia interpunctella. Physiol Entomol 3:135–146
Acknowledgments
We thank the Smithsonian Tropical Research Institute and Freie Universität Berlin for financial support of this work. Franziska Beran is grateful for the Short Term Fellowship awarded from the Smithsonian Tropical Research Institute in 2011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Franziska Beran and Sven Geiselhardt contributed equally
Rights and permissions
About this article
Cite this article
Beran, F., Geiselhardt, S., Vargas, G. et al. Cuticular Extracts from Acromis sparsa (Coleoptera: Cassidinae) Mediate Arrestment Behavior of the Commensal Canestriniid Mite Grandiella rugosita . J Chem Ecol 40, 996–1002 (2014). https://doi.org/10.1007/s10886-014-0494-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-014-0494-1