Abstract
This study investigated the roles of jasmonates in the regulation of sorgoleone accumulation and the expression of genes involved in sorgoleone biosynthesis in sorghum roots. Both methyl jasmonate (MeJa) and jasmonic acid (JA) substantially promoted root hair formation, secondary root development, root weight, and sorgoleone accumulation in sorghum roots. Sorgoleone content varied widely depending on the concentration of JA or MeJa and the duration of their application. Root weight and sorgoleone accumulation were highest after the application of JA or MeJa at a concentration of 5.0 μM, and then declined with increasing concentrations of jasmonates. At 5.0 μM, JA and MeJa increased sorgoleone content by 4.1 and 3.4-fold, respectively. Transcript accumulation was apparent for all genes, particularly for the O-methyltransferase 3 gene, which increased in expression levels up to 8.1-fold after a 36-h exposure to MeJa and 3.5-fold after a 48-h exposure to JA. The results of this study pave the way for more effective biosynthesis of sorgoleone, an important and useful allelochemical obtained from a variety of plant species.
Similar content being viewed by others
Introduction
The lipid benzoquinone sorgoleone (2-hydroxy-5-methoxy-3-[(8′Z, 11′Z)-8′, 11′, 14′-pentadecatriene]-p-benzoquinone) is a potent allelochemical that is produced as an oily exudate from the root hairs of sorghum (Sorghum bicolor) (Czarnota et al. 2001; Dayan et al. 2007), along with a variety of structural analogues (Kagan et al. 2003) responsible for inhibiting weeds and harmful algae (Einhellig and Souza 1992; Nimbal et al. 1996; Rimando et al. 2003; Uddin et al. 2009, 2012; Weston and Czarnota 2001). Sorgoleone is released from the roots of sorghum continually during the growing season, which may prolong its presence in soil (Dayan 2006; Dayan et al. 2009; Weidenhamer 2005). Sorgoleone, its resorcinol analogue, and other related hydroquinones are produced solely by living root hairs and are exuded as golden-coloured droplets from the tips of root hairs (Czarnota et al. 2003a; Dayan et al. 2009). Factors that affect root hair production and sorgoleone biosynthesis are not well understood. However, sorgoleone biosynthesis is linked intrinsically to the presence of living root hairs (Czarnota et al. 2001; Yang et al. 2004).
Plants use a variety of sequestration and transport mechanisms to move and export bioactive products safely into the rhizosphere (Weston et al. 2012). Specific root exudates and rhizodeposits are important messengers for chemical communication systems that help regulate the interactions between roots and soil organisms, and mediate processes in response to environmental stressors (Uren 2000). Roots, when they are under stress or encountering challenges in the rhizosphere, often react by releasing low-molecular-weight compounds, including amino acids, organic acids, and phenolics, as well as proteins. These secretions can protect the plant, thus initiating a negative form of communication in the rhizosphere (Bertin et al. 2003). Alternatively, these exudates can elicit symbiotic responses that initiate legume Rhizobium nitrogen fixation or attract common soil microbes, which are positive forms of communication (Mathesius and Watt 2011; Peters et al. 1986; Shi et al. 2011)
Sorgoleone biosynthesis appears to occur exclusively in root hair cells, which in sorghum appear as cytoplasmically dense cells filled with numerous osmiophilic deposits (Czarnota et al. 2003a). These deposits presumably are associated with the rhizosecretion of sorgoleone, which can constitute as much as 85 % of the exudate dry weight in some cultivars (Bertin et al. 2003; Czarnota et al. 2001, 2003b; Duke 2003; Rimando et al. 2003). The cell-specific localization and prolific output of the sorgoleone biosynthetic pathway renders the use of expressed sequence tag (EST) analysis an obvious method to isolate genes encoding sorgoleone biosynthetic enzymes (Baerson et al. 2008). Labeling studies performed by Fate and Lynn (1996) first demonstrated that biosynthesis proceeds through the action of an alkylresorcinol synthase (ARS), a novel type III polyketide synthase that acts on fatty acyl-CoA starter units (Baerson et al. 2008). Subsequently, both the predicted 5-n-pentadecatrienyl resorcinol and a 3-methyl ether derivative of sorgoleone were identified in sorghum root extracts, indicating that dihydroxylation of the resorcinol ring is preceded by O-methylation at the 3-hydroxyl position (Baerson et al. 2008; Dayan et al. 2003; Fate and Lynn 1996). Once released into the rhizosphere, the resulting chemically unstable hydroquinone rapidly oxidizes to the bioactive sorgoleone benzoquinone, where it may persist in soil for extended periods (Czarnota et al. 2001; Einhellig and Souza 1992; Gimsing et al. 2009; Netzly et al. 1988). Synthesis of sorgoleone from the available palmitoleoyl-CoA would, therefore, likely require, at minimum, the participation of a Δ12 and Δ15 fatty acid desaturase (DES), an ARS, 3-O-methyltransferase (OMT), and cytochrome P450 (Baerson et al. 2008) (Fig. 1).
Proposed biosynthestic pathway of the allelochemical, sorgoleone (Baerson et al. 2008)
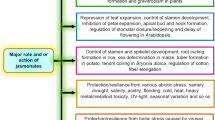
Root hair development has been used as an important model to study the mechanisms of cell patterning and differentiation in higher plants (Schiefelbein 2000). Mutational analysis has revealed that several genes in Arabidopsis function in the specification of root epidermal cell types. Among these, the TTG (Galway et al. 1994), GL2 (Masucci and Schiefelbein 1996), and WER (Wada et al. 1997) genes are the best characterized. Hormones are involved in the regulation of root hair development. Studies on Arabidopsis mutants, such as axr1 and ctr1, indicate that auxin and ethylene function in root hair initiation. Both methyl jasmonate (MeJa) and jasmonic acid (JA) have a pronounced effect on promoting root hair formation in Arabidopsis. The effect of MeJa and JA on root hair formation is blocked by ethylene inhibitors Ag+ or aminoethoxyvinylglycine (AVG). The stimulatory effects of MeJa and JA also are diminished in ethylene-insensitive mutants etr1-1 and etr1-3 (Zhu et al. 2006).
To the best of our knowledge, the effects of jasmonates on gene expression in sorghum roots have not been reported. The goal of this study was to correlate gene expression levels with the biosynthesis of sorgoleone after exposure to JA or MeJa for different time periods. Such knowledge may be useful for the future development of a bioherbicide by increasing levels of sorgoleone through molecular genetic manipulation.
Materials and Methods
Plant Material and Growth Conditions
Sorghum seeds of the cultivar Chalsusu, which has a high sorgoleone content (Uddin et al. 2009), were used. Seeds were treated with benomyl (a powdery fungicide, 100 g dissolved in 20 L) for 4 h, and then rinsed several times in distilled water. Seeds (25/Petri dish) were placed on the surface of sterile Whatman #1 filter paper (diam, 90 mm) saturated with different concentrations of JA or MeJa placed in sterile Petri dishes (100 × 40 mm). The dishes were then placed in a growth chamber at 25 °C under standard cool white fluorescent tubes with a flux rate of 550 μmol s−1 m−2 and a 16-h photoperiod for 1 week.
Application of MeJa and JA
To determine the concentrations of JA and MeJa that promote maximum sorgoleone biosynthesis, sorghum seeds were imbibed with solution containing different concentrations (0, 0.5, 1.0, 5.0, 10, 50, 100, 250, and 500 μM) of either JA or MeJa for 1 week. To examine how gene expression and sorgoleone accumulation in response to MeJa and JA changed over time, 5-day-old sorghum seedlings were treated once with 5.0 μM JA or MeJa, and then their roots were harvested for analysis at multiple time points (0, 3, 6, 12, 24, 36, 48, 60, and 72 h after treatment). Roots samples were stored in sealed clear polyethylene plastic bags at −80 °C until use. There were four replicates for each time point, and the entire experiment was repeated twice.
Extraction Procedure and Sorgoleone Analysis by HPLC
Sorgoleone was extracted according to the procedures described previously (Czarnota et al. 2003b; Netzly and Butler 1986; Netzly et al. 1988), except that methanol was used as a solvent instead of methylene chloride (Uddin et al. 2010). Seedling roots were excised and immersed in methanol (1:20 w/v) for 30 s to extract. The crude extract was filtered and evaporated under vacuum. The dried extract was dissolved in methanol (1 mg ml−1), and the solution was filtered through a poly filter (pore size, 0.45 μm). The filtrate was diluted 4-fold with methanol prior to HPLC analysis. HPLC quantification of sorgoleone was performed using the Futecs NS-4000 HPLC system (Futecs Co. Ltd., Daejeon, Korea), with a C18 column (250 × 4.6 mm, particle size 5 μm; RStech, Daejeon, Korea). The mobile phase was 75 % acetonitrile+25 % acidified water. Water was acidified with glacial acetic acid (97.5:2.5 v/v). Sorgoleone was detected at 280 nm with a Waters tunable absorbance detector after injection of 20 μl of the methanol solubilized crude extract sample. The column flow rate was 1 ml min−1 with a 40 min total run time for each sample. All samples were run in triplicate. The amount of sorgoleone was calculated on the basis of a standard curve obtained from a purified sample. The sorgoleone standard was provided by Franck Dayan, United States Department of Agriculture-Agricultural Research Service (USDA-ARS), Natural Products Utilization Research Unit.
Root Sample for Gene Expression
From the MeJa and Ja treatment root samples were collected for gene expression. Only the most optimal concentrations of MeJa and Ja (5.0 μM) for maximum sorgoleone concentration were selected.
RNA Extraction and cDNA Synthesis
Total RNA was isolated from a root of S. bicolor using tri-reagent (MRC, USA) and RNeasy Plant Mini Kit (QIAGEN, Valencia, CA, USA). For Quantitative real-time polymerase chain reaction (qRT-PCR), 1 μg of total RNA was reverse-transcribed according to the manufacturer’s protocol (ReverTraAce-a, TOYOBO, Japan) using an oligo (dT)20 primer. The cDNA mixtures were used as templates for qRT-PCR.
Real-Time Quantitative PCR
For transcript-level analysis by qRT-PCR, RNAs from auxin treated sorghum roots were harvested, and single-stranded cDNAs were synthesized from the isolated total RNA by using the aforementioned protocols. The gene-specific primer sets were designed for qRT-PCR (Table 1). Specific primers for DES2 (EF206347), DES3 (EF206348) (Pan et al. 2007), ARS1 (XM_002441794), ARS2 (XM_002449699) (Cook et al. 2010), and OMT3 (EF189708) (Baerson et al. 2008) were obtained from NCBI. Ubiquitin was used as control (Baerson et al. 2008). The PCRs were carried out in triplicates for 40 cycles on a MiniOpticon (Bio-Rad Laboratories, Hercules, CA, USA) using the QIAGEN Quantitect SYBR Green PCR Kit. The PCRs were pre-denatured at 95 °C for 5 min, then subjected to 40 cycles of amplifications with the following thermal cycles: denaturation for 30 s at 95 °C, annealing for 30 s at 55 °C; extension for 30 s at 72 °C. Fluorescent intensity data were acquired during extension step. The transcript levels were checked using a standard curve. Identical PCR conditions were used for all targets.
Statistical Analysis
All data were analyzed using the SAS 9.2 Software (SAS 2010; SAS Institute Inc., Cary, NC, USA). Analysis of variance was performed and means were separated by Duncan Multiple Range Test (DMRT) Standard deviations were provided to indicate the variation associated with a particular mean value.
Results
Root Growth and Root Hair Formation in Response to Jasmonates
The root growth pattern, secondary root, root hair formation, and root growth of sorghum were influenced by jasmonates. Photographs of sorghum root revealed variation in rooting patterns among the treatments (Figs. 2 and 3). The number of secondary or branching roots was higher at lower concentrations of jasmonate treatment, and the highest number of secondary roots was observed at 5 μM (Fig. 4). JA treatment produced a slightly higher number of secondary roots compared with that induced by MeJa treatment. Figure 5 shows clearly that root hair formation varied among the treatments. Greater numbers of healthy root hairs were formed in response to JA and MeJa treatments compared with those formed in the control. Root weight increased slowly with increases in the concentration of jasmonates (JA and MeJa) up to 5.0 μM, and then declined slowly with further increasing concentrations of either compound (Fig. 6).
Effects of jasmonates for the production of secondary roots in sorghum roots. Each point is the mean of four replicates with an average of 10 seedlings for each replicate. JA, jasmonic acid, MeJa, methyl jasmonate. Means with the same letter adjacent to the symbols are not different at P<0.05 according to a Duncan Multiple Range Test
Dose-response curves showing the effects of jasmonates on root growth. Each point is the mean of four replicates with an average of 10 seedlings for each replicate. JA, jasmonic acid, MeJa, methyl jasmonate. Means with the same letter adjacent to the symbols are not different at P < 0.05 according to a Duncan Multiple Range Test
Effect of Different Concentrations of Jasmonates on Sorgoleone Accumulation
The dose response experiment indicated that sorgoleone content varied widely depending on the concentrations of exogenous jasmonates (Fig. 7). Root dry weight and levels of sorgoleone increased with increases in both JA and MeJa concentrations up to 5.0 μM, and then declined with increasing concentrations of either of the jasmonates. Compared with untreated controls, sorgoleone levels were 4.1, 2.3, and 1.7-fold higher after treatment with 5, 1.0, and 0.5 μM of JA, respectively (Fig. 7). The level of sorgoleone was highest at a concentration of 5.0 μM JA, with a level approximately 10 times lower (2.8 mg/g root fresh weight) in roots exposed to 500 μM JA.
Dose-response curves showing the effects of jasmonates on sorgoleone production. Each point is the mean of four replicates with an average of 10 seedlings for each replicate. JA, jasmonic acid, MeJa, methyl jasmonate. Means with the same letter adjacent to the symbols are not different at P < 0.05 according to a Duncan Multiple Range Test
The concentration-dependent effects of MeJa on sorgoleone accumulation were almost identical for MeJa, with 5.0 μM MeJa producing more sorgoleone than any other concentration. Compared with an untreated control, sorgoleone levels were 3.4, 2.1, and 1.8-fold higher after exposure to 5, 1.0, and 0.5 μM of exogenous MeJa, respectively (Fig. 7). Exposure to concentrations of MeJa greater than 5.0 μM caused a less dramatic decrease in sorgoleone content than observed for JA (Fig. 7). After exposure to 500 μM of MeJa, the level of sorgoleone was 5.0 mg/g root fresh weight, which is 4.6 times lower than the highest content of sorgoleone observed in roots incubated with 5 μM of MeJa.
Effect of Length of Exposure to Optimal Jasmonate Concentration on Sorgoleone Content
Sorgoleone accumulation was maximal after treatment with 5.0 μM of either JA or MeJa, with the next highest levels of accumulation observed following treatments with jasmonates at 1.0 μM and 0.5 μM, respectively (Fig. 7). In the case of exposure to 5.0 μM MeJa, increasing exposure time caused sorgoleone levels to increase for up to 72 h after the beginning of the treatment (Fig. 8). Compared with the control at 0 h, 5.0 μM MeJa produced 2.3 times more sorgoleone 72 h after the beginning of the treatment. In the case of exposure to 5.0 μM JA, increasing exposure time caused sorgoleone levels to increase for up to 60 h after the beginning of the treatment (Fig. 8). Compared with the control at 0 h, 5.0 μM JA produced almost twice as much sorgoleone at 60 h after treatment. Levels of sorgoleone in control seedlings also increased with time. Sorgoleone levels were highest at 24 h, when they were 1.2 times higher than levels in control plants at 0 h.
Time-courses of sorgoleone production induced by application of jasmonates (5.0 µM) to sorghum roots. Each point is the mean of four replicates with an average of 10 seedlings for each replicate. JA, jasmonic acid, MeJA, methyl jasmonate. Means with the same letter adjacent to the symbols are not different at P < 0.05 according to a Duncan Multiple Range Test
Inducible Expression of Sorgoleone Biosynthetic Genes in Response to MeJa and JA
The effects of JA and MeJa on the levels of transcript of five genes involved in sorgoleone biosynthesis were monitored. Levels of transcript of the sorgoleone biosynthetic genes, DES2, DES3, ARS1, ARS2, and OMT3 increased after exposure to either JA or MeJa at 5.0 μM. The extent to which the levels of transcript were affected by either JA or MeJa was dependent on the length of time the plants were exposed to jasmonates.
Shortly after the application of JA, levels of transcripts were comparable to those in control seedlings, although higher transcript levels were noted than in control seedlings at some subsequent time points (Fig. 9). The time after initial exposure when transcript levels were highest differed among the genes. In the case of ARS1, the highest levels of transcript accumulation occurred 48 h after initial exposure. At 48 h levels of ARS1 transcript were 1.8-fold higher than in untreated seedlings at 0 h (Fig. 9). Levels of ARS2 and DES2 transcript were highest 36 h after treatment, with increases of 5.2 and 3.3-fold, respectively when compared with untreated seedlings at 0 h (Fig. 9). The largest increase in transcript accumulation was apparent for the DES3 gene, 12 h after JA treatment, when DES3 transcripts were 3.2-fold more abundant than in untreated seedlings at 0 h (Fig. 9).
Time course of induction of sorgoleone biosynthetic genes in response to jasmonic acid at 5.0 µM in the sorghum root. Each value is the mean of 3 replicates. Con, control; JA, jasmonic acid. Means with the same letter in both column bars are not different at P < 0.05 according to a Duncan Multiple Range Test
Transcripts of different sorgoleone biosynthetic genes responded differently to exposure to MeJa. The times when the largest increase in transcript abundance was apparent after treatment with JA differed among the genes. Gene expression was lower than in the untreated control shortly after initial exposure of sorghum seeds to MeJa. Compared with transcript levels in seedlings not treated with MeJa, transcript accumulation was highest for most of the genes 36 h after MeJa treatment (Fig. 10), in particular for OMT3 (increased up to 8.1-fold), DES2 (increased up to 5.2-fold), ARS2 (increased up to 2.8-fold), and DES3 (increased up to 2.4-fold). Levels of DES2 transcript were 4.1-fold higher 3 h after treatment with MeJa than in seedlings that received no MeJa (control). The highest level of ARS1 transcript was observed 24 h after treatment with MeJA, and was 3.7-fold higher than the level of ARS1 transcript in seedlings not treated with MeJa (0 h) (Fig. 10).
Time course of induction of sorgoleone biosynthetic genes by application of methyl jasmonate at 5.0 µM treatment in the sorghum root. Each value is the mean of 3 replicates. Con, control; MeJa, methyl jasmonate. Means with the same letter in both column bars are not different at P<0.05 according to a Duncan Multiple Range Test
Discussion
Plant hormones are involved in the regulation of root hair development. Several possible mechanisms might account for the increase in root hair density (Ma et al. 2001). First, more trichoblast files may be initiated. Second, increased abundance of trichoblasts with branched root hairs may contribute to an increase in root hair density. Third, atrichoblasts may be recruited into trichoblasts during root hair development under certain conditions. These three processes may occur either in isolation or in combination. Our results suggest that the initiation of recruitment of more trichoblast cells into root hairs may be an important cause of MeJa-induced root hair development. The induction of branched hair formation may be a novel effect of MeJa on root hair development. Investigation of whether JAs activate root hair formation via ethylene in Arabidopsis revealed that the effects of JAs were abolished in the ethylene-insensitive mutants etr1-1 and etr1-3, or by inhibitors of ethylene action (Ag+) or biosynthesis inhibitors (AVG). Furthermore, ibuprofen and SHAM, which inhibit JA biosynthesis, repressed ACC-driven or eto1-1-induced root hair formation. Collectively, these reports support a role for the interaction between JAs and ethylene in the regulation of root hair development in Arabidopsis. Czarnota et al. (2003a) confirmed that sorghum root hairs are physiologically active, and that they contain a complex network of smooth endoplasmic reticulum and possibly Golgi bodies. Small globules of cytoplasmic exudates also were observed to deposit an oily material between the cell wall and the plasma membrane near the root hair tips. Hess et al. (1992) indicated that sorgoleone production is quite sensitive to environmental conditions, and it is well documented that sorgoleone production depends mainly on root hair formation (Czarnota et al. 2003b; Dayan 2006; Dolan 2001; Yang et al. 2004).
Herein we report that root growth pattern, secondary root, root hair formation, and root growth of sorghum are influenced by the use of jasmonates. Our study shows that sorghum roots develop many branches (Fig. 4), as well as more root hairs (Fig. 5), when the proper concentrations of jasmonates (up to 5 μM) are applied. Clearly, healthy root hairs in higher numbers than those in the control were formed in response to JA and MeJa treatment. Root weight increased slowly with increased concentrations of jasmonates (JA and MeJa) up to 5.0 μM and then declined slowly with further increasing concentrations of these compounds (Fig. 6). Zhu et al. (2006) have reported that jasmonates promote root hair formation in Arabidopsis, which agrees with our findings.
Jasmonates are a class of plant hormones that mediate various aspects of gene and metabolic regulation, defense, stress responses, reproduction, and possibly communication. The role of JA in plants has received considerable attention, and various modes of action for JA and its methyl ester (MeJa) have been proposed (Farmer 2007; Farmer and Ryan 1990). JA is a small lipid precursor of jasmonate hormones. Farmer (2007), working largely with the model plant Arabidopsis thaliana, concluded that the two groups take different routes to the genes involved. Thines et al. (2007) analyzed eight related gene transcripts of unknown function that are rapidly upregulated in developing Arabidopsis stamens treated with jasmonates. By contrast, Chini et al. (2007) characterized a mutant called jasmonate-insensitive3-1 (jai3-1). Working with different genes, both groups found that over-expression of the full-length transcripts had little or no effect on jasmonate perception. However, over-expression of shortened forms that encode proteins truncated at the carboxyl terminus reduced sensitivity to jasmonates. Additionally, although full-length proteins were unstable in vivo when treated with jasmonates, proteins with a truncated carboxyl terminus resisted degradation in response to such treatment. Doornbos et al. (2011) reported that induced systemic resistance (ISR) occurs in response to the application of MeJa, and systemic acquired resistance (SAR) occurs after treatment with salicylic acid or benzothiadiazole, whereas some of the Arabidopsis genotypes affected in defense signaling show altered numbers of culturable bacteria in their rhizospheres; however, the effects depend on soil type.
Genes involved in jasmonate biosynthesis, secondary metabolism, and cell-wall formation, and those encoding stress-protective and defense proteins are up-regulated by MeJA treatment (Cheong and Choi 2003). In this study, five transcripts-DES2, DES3, ARS1, ARS2, and OMT3-that encode enzymes involved in sorgoleone biosynthesis were shown to accumulate in response to treatment with JA and MeJa. Transcript accumulation was apparent for all genes (Figs. 9 and 10), particularly OMT3. Levels of OMT3 transcript accumulated to levels 8.1-fold higher than in seedlings not treated with MeJa. This observation is supported by other reported documents (Bonfill et al. 2011; Kim et al. 2009; Korsangruang et al. 2010; Lystvan et al. 2010; Rhee et al. 2010), where they investigated the capability of JA and its methyl ester, MeJa, to induce the accumulation of various secondary metabolites, and showed higher levels of expression for their respective genes and also from previous work (Uddin et al. 2011), where they observed that sorgoleone biosynthetic genes are up or down-regulated by auxins. The application of phytohormones to excised sections or organs (e.g., root, elongating region of hypocotyl, basal region of hypocotyl, and plumule) of intact soybean seedlings resulted in the accumulation of mRNAs for pGHl-4 to levels that are many-fold greater than those found in untreated organs of seedlings (Hagen and Guilfoyle 2002).
The results obtained using jasmonates to enhance sorgoleone production suggest that sorghum roots benefit from exposure to jasmonates by developing more sorgoleone-rich root hairs, showing different rooting pattern and root weight variation among the jasmonate treatments. Transcript accumulation was apparent for all of the seven genes tested, particularly OMT3, for which transcript levels increased up to 8.1-fold after a 36 h exposure to MeJa compared with OMT3 transcript levels in untreated seedlings. In summary, these findings suggested that jasmonates (JA and MeJa) are potent substances for promoting root hair formation and sorgoleone accumulation and up-regulating gene expression in sorghum. Further research should focus on the involvement of these products in the regulation of root responses to stress.
References
Baerson SR, Dayan FE, Rimando AM, Nanayakkara NP, Liu CJ, Schroder J, Fishbein M, Pan Z, Kagan IA, Pratt LH, Cordonnier-Pratt MM, Duke SO (2008) A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs. J Biol Chem 283:3231–3247
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Bonfill M, Mangas S, Moyano E, Cusido RM, Palazón J (2011) Production of centellosides and phytosterols in cell suspension cultures of Centella asiatica. Plant Cell Tissue Organ Cult 104:61–67
Cheong JJ, Choi YD (2003) Methyl jasmonate as a vital substance in plants. Trends Genet 19:409–413
Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, García-Casado G, López-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R (2007) The JAZ family of repressors is the missing link in jasmonate signaling. Nature 448:666–671
Cook D, Rimando AM, Clemente TE, Schroder J, Dayan FE, Nanayakkara D, Pan Z, Noonan BP, Fishbein M, Abe I, Duke SO, Baerson SR (2010) Alkylresorcinol synthases expressed in Sorghum bicolor root hairs play an essential role in the biosynthesis of the allelopathic benzoquinone sorgoleone. Plant Cell 22:867–887
Czarnota MA, Paul RN, Dayan FE, Nimbal CI, Weston LA (2001) Mode of action, localization of production, chemical nature, and activity of sorgoleone: a potent PSII inhibitor in Sorghum spp. root exudates. Weed Technol 15:813–825
Czarnota MA, Paul RN, Weston LA, Duke SO (2003a) Anatomy of sorgoleone-secreting root hairs of Sorghum species. Int J Plant Sci 164:861–866
Czarnota MA, Rimando AM, Weston LA (2003b) Evaluation of seven sorghum (Sorghum sp.) accessions. J Chem Ecol 29:2073–2083
Dayan FE (2006) Factors modulating the levels of the allelochemical sorgoleone in Sorghum bicolor. Planta 224:339–346
Dayan FE, Kagan IA, Rimando AM (2003) Elucidation of the biosynthetic pathway of the allelochemical sorgoleone using retrobiosynthetic NMR analysis. J Biol Chem 278:28607–28611
Dayan FE, Watson SB, Nanayakkara NPD (2007) Biosynthesis of lipid resorcinols and benzoquinones in isolated secretory plant root hairs. J Exp Bot 58:3263–3272
Dayan FE, Weidenhamer JD, Howell J (2009) Dynamic root exudation of sorgoleone and its in planta mechanism of action. J Exp Bot 60:2107–2117
Dolan L (2001) The role of ethylene in root hair growth in Arabidopsis. J Plant Nutr Soil Sci 164:141–145
Doornbos RF, Geraats BP, Kuramae EE, Van Loon LC, Bakker PA (2011) Effects of jasmonic acid, ethylene, and salicylic acid signaling on the rhizosphere bacterial community of Arabidopsis thaliana. Mol Plant Microbe Interact 24:395–407
Duke SO (2003) Weeding with transgenes. Trends Biotechnol 21:192–195
Einhellig FA, Souza IF (1992) Phytotoxicity of sorgoleone found in grain sorghum root exudates. J Chem Ecol 18:1–11
Farmer EE (2007) Plant biology—jasmonate perception machines. Nature 448:659–660
Farmer EE, Ryan CA (1990) Interplant communication—airborne methyl jasmonate induces synthesis of proteinase-inhibitors in plant-leaves. Proc Natl Acad Sci USA 87:7713–7716
Fate GD, Lynn DG (1996) Xenognosin methylation is critical in defining the chemical potential gradient that regulates the spatial distribution in Striga pathogenesis. J Am Chem Soc 118:11369–11376
Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW (1994) The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Dev Biol 166:740–754
Gimsing AL, Baelum J, Dayan FE, Locke MA, Sejero LH, Jacobsen CS (2009) Mineralization of the allelochemical sorgoleone in soil. Chemosphere 76:1041–1047
Hagen G, Guilfoyle TJ (2002) Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49:373–385
Hess DE, Ejeta G, Butler LG (1992) Selecting sorghum genotypes expressing a quantitative biosynthetic trait that confers resistance to Striga. Phytochemistry 31:493–497
Kagan IA, Rimando AM, Dayan FE (2003) Chromatographic separation and in vitro activity of sorgoleone congeners from the roots of Sorghum bicolor. J Agric Food Chem 51:7589–7595
Kim OT, Bang KH, Kim YC, Hyun DY, Kim MY, Cha SW (2009) Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tissue Organ Cult 98:25–33
Korsangruang S, Soonthornchareonnon N, Chintapakorn Y, Saralamp P, Prathanturarug S (2010) Effects of abiotic and biotic elicitors on growth and isoflavonoid accumulation in Pueraria candollei var. candollei and P. candollei var. mirifica cell suspension cultures. Plant Cell Tissue Organ Cult 103:333–342
Lystvan K, Belokurova V, Sheludko Y, Ingham JL, Prykhodko V, Kishchenko O, Paton E, Kuchuk M (2010) Production of bakuchiol by in vitro systems of Psoralea drupacea Bge. Plant Cell Tissue Organ Cult 101:99–103
Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ 24:459–467
Masucci JD, Schiefelbein JW (1996) Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell 8:1505–1517
Mathesius U, Watt M (2011) Rhizosphere signals for plant-microbe interactions: implications for field-grown plants. In: Lüttge U, Beyschlag W, Büdel B, Francis D (eds) Progress in botany 72. Springer, Berlin, pp 125–161
Netzly DH, Butler LG (1986) Roots of sorghum exude hydrophobic droplets containing biologically active components. Crop Sci 26:775–778
Netzly DH, Riopel JL, Ejeta G, Butler LG (1988) Germination stimulants of witchweed (Striga asiatica) from hydrophobic root exudates of sorghum (Sorghum bicolor). Weed Sci 36:441–446
Nimbal CI, Pedersen JF, Yerkes CN, Weston LA, Weller SC (1996) Phytotoxicity and distribution of sorgoleone in grain sorghum germplasm. J Agric Food Chem 44:1343–1347
Pan Z, Rimando AM, Baerson SR, Fishbein M, Duke SO (2007) Functional characterization of desaturases involved in the formation of the terminal double bond of an unusual 16:3Δ9, 12, 15 fatty acid isolated from Sorghum bicolor root hairs. J Biol Chem 282:4326–4335
Peters NK, Frost JW, Long SK (1986) A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233:977–980
Rhee HS, Cho HY, Son SY, Yoon SYH, Park JM (2010) Enhanced accumulation of decursin and decursinol angelate in root cultures and intact roots of Angelica gigas Nakai following elicitation. Plant Cell Tissue Organ Cult 101:295–302
Rimando AM, Dayan FE, Streibig JC (2003) PSII inhibitory activity of resorcinolic lipids from Sorghum bicolor. J Nat Prod 66:42–45
SAS (2010) The Little SAS Book for Enterprise Guide 4.2. 294–295. Statistical Analysis Systems Institute, Cary, NC, USA
Schiefelbein JW (2000) Constructing a plant cell: the genetic control of root hair development. Plant Physiol 124:1525–1531
Shi S, Richardson AE, O’callaghan M, Deangelis KM, Jones EE, Stewart A, Firestone MK, Condron LM (2011) Effects of selected root exudate components on soil bacterial communities. FEMS Microbiol Ecol 77:600–610
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signaling. Nature 448:661–665
Uddin MR, Kim YK, Park SU, Pyon JY (2009) Herbicidal activity of sorgoleone from grain sorghum root exudates and its contents among sorghum cultivars. Kor J Weed Sci 29:229–236
Uddin MR, Park KW, Kim YK, Park SU, Pyon JY (2010) Enhancing sorgoleone levels in grain sorghum root exudates. J Chem Ecol 36:914–922
Uddin MR, Park WT, Kim YK, Pyon JY, Park SU (2011) Effects of auxins on sorgoleone accumulation and genes for sorgoleone biosynthesis in sorghum roots. J Agric Food Chem 59:12948–12953
Uddin MR, Min SK, Kim JD, Pyon JY (2012) Sorgoleone, a sorghum root exudate: algicidal activity and acute toxicity to the ricefish Oryzias latipes. Aquat Bot 98:40–44
Uren NC (2000) Types, amounts, and possible functions of compounds released into the rhizosphere by soil grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the plant-soil interface. Marcel Dekker, New York, pp 19–40
Wada T, Tachibana T, Shimura Y, Okada K (1997) Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 227:1113–1116
Weidenhamer J (2005) Biomimetic measurement of allelochemical dynamics in the rhizosphere. J Chem Ecol 31:221–236
Weston LA, Czarnota MA (2001) Activity and persistence of sorgoleone, a long-chain hydroquinone produced by Sorghum bicolor. J Crop Prod 4:363–377
Weston LA, Ryan PR, Watt M (2012) Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J Exp Bot 63(3445–3454):2012
Yang X, Owens TG, Scheffler BE, Weston LA (2004) Manipulation of root hair development and sorgoleone production in sorghum seedlings. J Chem Ecol 30:199–213
Zhu C, Gan L, Shen Z, Xia K (2006) Interactions between jasmonates and ethylene in the regulation of root hair development in Arabidopsis. J Exp Bot 57:1299–1308
Acknowledgements
This study was supported by Technology Development Program for Agriculture, Ministry of Agriculture, Forestry and Foods, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Uddin, M.R., Thwe, A.A., Kim, Y.B. et al. Effects of Jasmonates on Sorgoleone Accumulation and Expression of Genes for Sorgoleone Biosynthesis in Sorghum Roots. J Chem Ecol 39, 712–722 (2013). https://doi.org/10.1007/s10886-013-0299-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0299-7