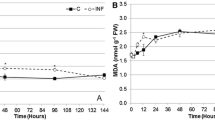
Abstract
Ascorbate is the major water-soluble antioxidant in plants and animals, and it is an essential nutrient for most insect herbivores. Therefore, ascorbate oxidase (AO) has been proposed to function as a plant defense that decreases the availability of ascorbate to insects. This hypothesis was tested by producing transgenic poplar (Populus tremula × Populus alba; Salicaceae) with 14- to 37-fold higher foliar AO activities than control (wild type) leaves and feeding these leaves to Lymantria dispar L. (Lepidoptera: Lymantriidae) caterpillars and Melanoplus sanguinipes (Fabricius) (Orthoptera: Acrididae) grasshoppers. To examine potential mechanisms of activity of AO in these insects, ascorbyl radical and/or ascorbate levels were measured in gut contents. No significant changes in ascorbyl radical or ascorbate levels were found in the midgut contents of L. dispar larvae that ingested the leaves of the AO-overexpressing genotypes compared to the control genotype, and no significant decreases in ascorbate levels were found in the foregut or midgut contents of M. sanguinipes. Treatment of control leaves with commercial AO also produced no changes in the midgut biochemistry of L. dispar larvae, as measured by levels of ascorbyl radicals. Likewise, no increase in oxidative stress was observed in L. dispar that consumed tannin-treated AO-overexpressing leaves compared with tannin-treated control genotype leaves. Performance experiments were carried out on first- and fourth-instar L. dispar larvae on leaf disks and on third instars feeding on intact leaves on trees. In no case was a significant difference found in the contrast between the control and three AO-overexpressing genotypes for relative consumption rate, relative growth rate, or nutritional indices. We conclude that elevated levels of AO in poplar are unlikely to serve as a defense against herbivores such as L. dispar or M. sanguinipes and that the low oxygen levels commonly found in the guts of caterpillars and grasshoppers may limit the activity of ingested AO in these leaf-chewing insects.




Similar content being viewed by others
References
An, G., Ebert, P. R., Mitra, A., and Ha, S. B. 1988. Binary vectors, pp. 1–19, in S. B. Gelvin, and R. A. Schilperoort (eds.). Plant Molecular Biology Manual. Kluwer, Dordrecht, The Netherlands.
Appel, H. M., and Maines, L. W. 1995. The influence of host plant on gut conditions of gypsy moth (Lymantria dispar) caterpillars. J. Insect Physiol. 41:241–246.
Barbehenn, R. V. 2003. Antioxidants in grasshoppers: higher levels defend the midgut tissues of a polyphagous species than a graminivorous species. J. Chem. Ecol. 29:683–702.
Barbehenn, R. V., Martin, M. M., and Hagerman, A. E. 1996. Reassessment of the roles of the peritrophic envelope and hydrolysis in protecting polyphagous grasshoppers from ingested hydrolyzable tannins. J. Chem. Ecol. 22:1901–1919.
Barbehenn, R. V., Bumgarner, S. L., Roosen, E., and Martin, M. M. 2001. Antioxidant defenses in caterpillars: role of the ascorbate recycling system in the midgut lumen. J. Insect Physiol. 47:349–357.
Barbehenn, R. V., Poopat, U., and Spencer, B. 2003. Semiquinone and ascorbyl radicals in the gut fluids of caterpillars measured with EPR spectrometry. Insect Biochem. Mol. Biol. 33:125–130.
Barbehenn, R. V., Cheek, S., Gasperut, A., Lister, E., and Maben, R. 2005a. Phenolic compounds in red oak and sugar maple leaves have prooxidant activities in the midguts of Malacosoma disstria and Orgyia leucostigma caterpillars. J. Chem. Ecol. 31:969–988.
Barbehenn, R., Dodick, T., Poopat, U., and Spencer, B. 2005b. Fenton-type reactions and iron concentrations in the midgut fluids of tree-feeding caterpillars. Arch. Insect Biochem. Physiol. 60:32–43.
Barbehenn, R. V., Jones, C. P., Yip, L., Tran, L., and Constabel, C. P. 2007. Limited impact of elevated levels of polyphenol oxidase on tree-feeding caterpillars: assessing individual plant defenses with transgenic poplar. Oecologia 154:129–140.
Bi, J. L., and Felton, G. W. 1995. Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J. Chem. Ecol. 21:1511–1530.
Bi, J. L., Murphy, J. B., and Felton, G. W. 1997. Antinutritive and oxidative components as mechanisms of induced resistance in cotton. J. Chem. Ecol. 23:97–117.
Bolwell, G. P., and Wojtaszek, P. 1997. Mechanisms for the generation of reactive oxygen species in plant defence—a broad perspective. Physiol. Mol. Plant Pathol. 51:347–366.
Duffey, S. S., and Stout, M. J. 1996. Antinutritive and toxic components of plant defense against insects. Arch. Insect Biochem. Physiol. 32:3–37.
Felton, G. W., and Duffey, S. S. 1992. Ascorbate oxidation reduction in Helicoverpa zea as a scavenging system against dietary oxidants. Arch. Insect Biochem. Physiol. 19:27–37.
Felton, G. W., and Summers, C. B. 1993. Potential role of ascorbate oxidase as a plant defense protein against insect herbivory. J. Chem. Ecol. 19:1553–1568.
Fotopoulos, V., Sanmartin, M., and Kanellis, A. K. 2006. Effect of ascorbate oxidase over-expression on ascorbate recycling gene expression in response to agents imposing oxidative stress. J. Exp. Bot. 57:3933–3943.
Garcia-Pineda, E., Castro-Mercado, E., and Lozoya-Gloria, E. 2004. Gene expression and enzyme activity of pepper (Capsicum annuum L.) ascorbate oxidase during elicitor and wounding stress. Plant Sci. 166:237–243.
Gonzalez-Reyes, J. A., Alcain, F. J., Caler, J. A., Serrano, A., Cordoba, F., and Navas, P. 1995. Stimulation of onion root elongation by ascorbate and ascorbate free radical in Allium cepa L. Protoplasma 184:31–35.
Harris, M. D., Brewer, J. W., and Merril, L. D. 1980. Insect involvement in the transmission of bacterial pathogens, pp. 201–292, in K. F. Harris, and K. Maramorosch (eds.). Vectors of Plant Pathogens. Academic, New York, USA.
Hermsmeier, D., Schittko, U., and Baldwin, I. T. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and it natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 125:683–700.
Horton, D. R., and Redak, R. A. 1993. Further comments on analysis of covariance in insect dietary studies. Entomol. Exp. Appl. 69:263–275.
Johnson, K. S., and Barbehenn, R. V. 2000. Oxygen levels in the gut lumens of herbivorous insects. J. Insect Physiol. 46:897–903.
Karban, R., and Baldwin, I. T. 1997. Induced Responses to Herbivory. University of Chicago Press, Chicago, IL, USA.
Kato, N., and Esaka, M. 2000. Expansion of transgenic tobacco protoplasts expressing pumpkin ascorbate oxidase is more rapid than that of wild-type protoplasts. Planta 210:1018–1022.
Krishnan, N., Kodrik, D., Turanli, F., and Sehnal, F. 2007. Stage-specific distribution of oxidative radicals and antioxidant enzymes in the midgut of Leptinotarsa decemlineata. J. Insect Physiol. 53:67–74.
Larson, P. R., and Isebrands, J. G. 1971. The plastochron index as applied to developmental studies of cottonwood. Can. J. For. Res. 1:1–11.
Leplé, J. C., Brasileiro, A. C. M., Michel, M. F., Delmotte, F., and Jouanin, L. 1992. Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep 11:137–141.
Lindroth, R. L., and Weiss, A. P. 1994. Effects of ascorbic acid deficiencies on larvae of Lymantria dispar (Lepidoptera: Lymantriidae). Great Lakes Entomol. 27:169–174.
Maccarrone, M., D’Andrea, G., Salucci, M. L., Avigliano, L., and Finazzi-Agro, A. 1993. Temperature, pH and UV irradiation effects on ascorbate oxidase. Phytochemistry 32:795–798.
Major, I. T., and Constabel, C. P. 2006. Molecular analysis of poplar defense against herbivory. Comparison of wound- and insect elicitor-induced gene expression. New Phytol. 172:617–635.
Pignocchi, C., Fletcher, J. M., Wilkinson, J. E., Barnes, J. D., and Foyer, C. H. 2003. The function of ascorbate oxidase in tobacco. Plant Physiol. 132:1631–1641.
Pignocchi, C., Kiddle, G., Hernandez, I., Foster, S. J., Asensi, A., Taybi, T., Barnes, J., and Foyer, C. H. 2006. Ascorbate oxidase-dependent changes in the redox state of the apoplast modulate gene transcript accumulation leading to modified hormone signaling and orchestration of defense processes in tobacco. Plant Physiol. 141:423–435.
Ralph, S., Oddy, C., Cooper, D. et al. 2006. Genomics of hybrid poplar (Populus trichocarpa × deltoides) interacting with forest tent caterpillars (Malacosoma disstria): normalized and full-length cDNA libraries, expressed sequence tags, and cDNA microarray for the study of insect-induced defences in poplar. Mol. Ecol. 15:1275–1297.
Sambrook, J., and Russell, D. W. 2001. Molecular Cloning: A Laboratory Manual. 3rd edn.Cold Spring Harbor Press, Cold Spring Harbor, NY, USA.
Sanmartin, M., Drogoudi, P. D., Lyons, T., Pateraki, I., Barnes, J., and Kanellis, A. K. 2003. Over-expression of ascorbate oxidase in the apoplast of trangenic tobacco results in altered ascorbate and glutathione redox states and increased sensitivity to ozone. Planta 216:918–928.
Sanmartin, M., Pateraki, I., Chatzopoulou, F., and Kanellis, A. K. 2007. Differential expression of the ascorbate oxidase multigene family during fruit development and in response to stress. Planta 225:873–885.
SAS Institute. 2003. The SAS system for Windows. Version 9.1. SAS Institute, Cary, NC, USA.
Schultz, J. C., and Lechowicz, M. J. 1986. Hostplant, larval age, and feeding behavior influence midgut pH in the gypsy moth (Lymantria dispar). Oecologia 71:133–137.
Strothkamp, R. E., and Dawson, C. R. 1978. A kinetic study of the effects of hydrogen peroxide and pH on ascorbate oxidase. Biochem. Biophys. Res. Comm. 85:655–661.
Summers, C. B., and Felton, G. W. 1994. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuiidae): potential mode of action for phenolic compounds in plant anti-herbivore chemistry. Insect Biochem. Mol. Biol. 24:943–953.
Yamamoto, A., Bhuiyan, M. N. H., Waditee, R., Tanaka, Y., Esaka, M. et al. 2005. Suppressed expression of the apoplastic ascorbate oxidase gene increases salt tolerance in tobacco and Arabidopsis plants. J. Exp. Bot. 56:1785–1796.
Yoshimura, K., Ishikawa, T., Nakamura, Y., Tamoi, M., Takeda, T. et al. 1998. Comparative study on recombinant chloroplastic and cytosolic ascorbate peroxidase isozymes of spinach. Arch. Biochem. Biophys. 353:55–63.
Waldbauer, G. P. 1968. The consumption and utilization of food by insects. Adv. Insect Physiol. 5:229–289.
Wilkinson, L. 2000. SYSTAT: The system for statistics. SYSTAT, Evanston, IL, USA.
Acknowledgments
This project was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2004-35302-14840 to RVB and CPC. We thank Juha-Pekka Salminen for providing pedunculagin and pentagalloyl glucose, John Tanner for providing L. dispar eggs, Stefan Jaronski for providing M. sanguinipes eggs, Cara Mozola for measuring AO activity, Grace Lee for measuring ascorbate, and Robin Mellway for assistance in transforming the poplar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbehenn, R.V., Jaros, A., Yip, L. et al. Evaluating Ascorbate Oxidase as a Plant Defense Against Leaf-Chewing Insects Using Transgenic Poplar. J Chem Ecol 34, 1331–1340 (2008). https://doi.org/10.1007/s10886-008-9539-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9539-7