Abstract
To examine the feasibility, acceptability, and preliminary efficacy of a technology-assisted stepped-care behavioral intervention to improve adherence in adolescents with asthma. Thirty adolescents (Mage = 14.66, 53% male) with moderate to severe-persistent asthma completed daily adherence monitoring and medication reminders via a mobile app (Step 1). Participants with < 68% adherence during Step 1 received a telehealth behavioral intervention (Step 2). Twenty-six of 30 participants (87%) completed Step 1. Step 2 was indicated for 18 participants and was completed by 17. Participants favorably rated their experience in the study. Improvements in adherence (40–58%, p = .048) and decreases in asthma composite severity scores (CASI 6.08–5.08, p = .023) were observed for the full sample. Technology-assisted stepped-care is feasible and acceptable. Participants demonstrated improved adherence and asthma composite severity scores once they received the appropriate step of the intervention. Future studies should include a control group, a longer time-frame and an intermediate intervention step.
Similar content being viewed by others
Asthma is the most common childhood chronic illness, affecting approximately 6.1 million children and resulting in $5.9 billion in healthcare spending in the United States each year (Center for Disease Control and Prevention, 2016; Perry et al., 2019; Sullivan et al., 2017). Although pediatric asthma can be well-managed with a combination of medications, approximately half of adolescents report adherence rates of < 50% (Kaplan & Price, 2020; Morton et al., 2014). Improved adherence has been associated with improvements in asthma symptoms and exacerbations and overall health-related quality of life (Kaplan & Price, 2020; Lin et al., 2020).
Research has shown that digital interventions are preferred and show promise for improving adherence and other health outcomes (e.g., symptom control, lung function) in adolescents with asthma (Merchant et al., 2018; Ramsey et al., 2018, 2020). They also allow for the increased accessibility, customizability, and potential cost-effectiveness needed to improve treatment adherence in all adolescents with asthma. Most studies, however, do not assess adherence systematically using electronic monitoring, the gold standard for providing an objective measurement of adherence, (Jochman et al., 2017) or in a way that allows for intervention changes based on changes in adherence outcomes.
The combination of electronic monitoring of adherence and digitally delivered interventions allows for systematic assessment of adherence outcome and intervention customization from afar; therefore, a technology-assisted stepped-care intervention may be an optimal solution. Stepped-care interventions feature a hierarchy of interventions that individuals move through based on systematically assessed needs to ensure that individuals receive the lowest intensity treatment expected to be effective (e.g., less time/cost intensive) (Bower & Gilbody, 2005; Nicholas et al., 2019). The use of ongoing monitoring targets variability in individual behavior and has shown promise in promoting behavioral health in substance use disorders (Sobell & Sobell, 2000) and weight management interventions (Jakicic et al., 2012). Stepped-care is only beginning to be utilized in behavioral interventions and it has never been utilized to promote medication adherence in pediatric chronic illness management.
The primary aim of the present study was to assess the feasibility and acceptability of the first technology-assisted stepped-care intervention aimed at improving adherence. Our secondary aims were to examine changes in electronically monitored adherence and asthma composite severity scores, as well as the relationship between adherence, asthma composite severity scores, and lung function. An additional exploratory aim was to identify factors related to progression in the stepped-care intervention, including sociodemographic and disease-specific factors.
Methods
Procedures
The present study examined a stepped-care digital intervention aimed at improving inhaled controller medication adherence in adolescents ages 12–17 with moderate to severe-persistent asthma. The study utilized an AB (baseline and intervention) design to assess intervention feasibility, adherence via electronic monitoring, and asthma composite severity scores. IRB approval was obtained prior to recruitment. Inclusion criteria required that the adolescent: (1) had a physician-diagnosed moderate or severe-persistent asthma based on the National Asthma Education and Prevention Program guidelines (National Asthma Education & Prevention Program, 2007); (2) was prescribed at least one daily inhaled corticosteroid medication; (3) had a previous, valid pulmonary function test (PFT) with a forced vital capacity (FVC) of ≥ 1L; and (4) was between ages 12 and 17. Individuals with a significant cognitive impairment, serious mental illness, no English fluency, or a history of spirometry-induced bronchospasm were not eligible for participation. Patients were approached by a research team member during an outpatient pulmonary asthma clinic appointment. Interested families provided written assent/consent.
Baseline
Participants completed questionnaires with study staff. Participants received Cohero® mobile tracking sleeves (Cohero Health, Inc.) for their inhalers, as well as an Android smartphone with a prepaid data plan and the mobile applications required for the study. BreatheSmart™ app transmitted the adherence data from the Cohero mobile tracking sleeves and provided daily medication reminders throughout the study. Participants also completed an in-office spirometry test and received training from a respiratory therapist using ATS guidelines (Miller et al., 2005) on the use of a mobile spirometer. The MedaCheck Habit™ app sent push notifications to remind participants to provide weekly mobile spirometry readings for the duration of the study M(SD) = 4.48 months(1.58). Participants were contacted by study staff to assess monthly composite severity scores and asthma control.
Step 1: Digital Medication Reminders
Following a baseline period of four weeks, participants received Step 1, daily digital medication reminders through the MedaCheck Habit™ app. Adherence monitoring continued during Step 1, but no adherence feedback was provided. After four weeks of receiving digital medication reminders, participant adherence rates were calculated. Participants whose average adherence was below 68% qualified for the telehealth behavioral intervention (Step 2) as previous research has demonstrated 68.2% to be the adherence threshold below which children with asthma require oral corticosteroids (Milgrom et al., 1996). The average duration of Step 1 was eight weeks M(SD) = 8.23(3.73).
Step 2: Telehealth Behavioral Intervention
Step 2 consisted of four weekly, telehealth behavioral intervention sessions and access to adherence feedback via the BreatheSmart™ app. The manualized intervention sessions included self-monitoring strategies, discussions of individual barriers to adherence and allocation of treatment responsibility, organizational strategies to improve adherence, and guided problem-solving training individually tailored to the adolescent’s unique barriers to adherence. This intervention was adapted from a school-based telehealth adherence promotion intervention for adolescents with asthma (Lin et al., 2020). Intervention sessions were conducted with trained psychology doctoral trainees and included fidelity checks for the initial 10 sessions and 20% of sessions thereafter. Step 2 lasted approximately five weeks M(SD) = 5.23(1.76).
Measures
Demographic Information
Participants completed demographic questionnaires which were verified via chart review.
Feasibility and Acceptability
Feasibility was measured by calculating enrollment, retention, and participation rates over the course of the study. Acceptability assessed participant experience, satisfaction, helpfulness, ease of use, and potential future use of the intervention, apps, and mobile spirometer using a 12-item quantitative questionnaire (Lin et al., 2020). Items were scored on a 0–4 scale with higher scores indicating a more positive experience. Example items can be found in Fig. 1.
This figure depicts the average ratings provided by participants upon completion of the study when asked about their experience participating in the study. Participants were asked to respond to the questions listed on a scale of 0–4 with 0 indicating “Not at all” and four indicating “Extremely”. The last question was only answered by participants who received Step 2
Adherence
Cohero® electronic monitoring system, which includes a Bluetooth-enabled sensor sleeve that fits around the patient’s inhaler, was used to objectively assess adherence. Adherence data was sent to an online database accessible to study staff and adherence was calculated as a percentage based on prescribed dose (e.g., doses taken/doses prescribed × 100). Baseline adherence was calculated based on the last four weeks of baseline to allow for adjustment to the electronic monitoring system. Step 1 and 2 adherence was calculated from the initial four weeks of each intervention. Study end adherence was calculated from the last four weeks of the study.
Disease Severity and Control
Functional disease severity and control were assessed at enrollment and on a monthly basis with the composite asthma severity index (CASI) and asthma control test (ACT) (duRivage et al., 2017; Wildfire et al., 2012). A modified CASI asthma severity score combining emergency department visits, oral corticosteroid use, and symptoms was calculated via the TreatSmart program, a web-based software with lower scores indicating improved asthma control (Dexheimer et al., 2013; Lin et al., 2020). The ACT, a five-item measure of asthma severity, symptom frequency, symptom control, and inhaler use, was obtained as a measure of disease control (duRivage et al., 2017). Items are rated along a five-point Likert scale with higher scores indicating better asthma control.
Lung Function
Participants used a mobile spirometer (MIR Spirobank Smart™), an app-based bi-directional turbine system that recorded multiple parameters of lung function, including forced vital capacity (FVC) and forced expiratory volume (FEV1). The precision and accuracy of the MIR Spirobank Smart™ meet the European Respiratory Society (ERS) and American Thoracic Society (ATS) standards (Miller et al., 2005). Lung function values were accessible to study staff through a secure database and FEV1 was accessible to participants through the MedaCheck Habit™ app. The MedaCheck Habit app also provided specific feedback to participants to reperform mobile spirometry when readings were unacceptable. Only acceptable and physiologically possible efforts were included in statistical analyses. Lung function data from the mobile spirometer was reported as a percent predicted based on reference equations (Wang et al., 1993). Lung function data collected via mobile spirometry closest to the end of each study period were used for analyses.
Data Analytic Plan
All analyses were computed with IBM SPSS Version 26. Descriptive analyses were conducted to summarize demographic, feasibility, and acceptability data. In order to determine overall feasibility and acceptability, enrollment, retention, participation, and acceptability results were compared to a priori criteria (Lewis et al., 2021) based on previous research in this population (i.e., enrollment ≥ 70%, retention ≥ 80%, participation ≥ 80%, acceptability ≥ 3.0). Independent samples t-tests and analyses of variance were used to examine relationships among demographic variables and between those remaining in Step 1 and those whose adherence indicated stepping up to Step 2.
A target sample size of 30 was set to exceed the recommended sample size (n = 20) for a pilot trial designed to inform a larger efficacy trial with 80% power to detect a medium effect (Bell et al., 2018). Paired-samples t-tests were used to calculate the change in adherence, composite severity scores, control, and lung function as participants moved through the study. The total number of participants per analysis varied slightly due to missing adherence and lung function data. Missing adherence data rates were 22% for the baseline period, 7% for the step 1 intervention period, and 12% for the step 2 intervention period and were due to difficulties with syncing the monitoring devices. Pairwise deletion was used for these cases. Cohen’s d was calculated to estimate effect size and interpreted as small (.20), medium (.50), and large (.80) (Cohen, 1992). Bivariate correlations were conducted to examine the association between adherence, composite severity scores, control, and lung function at each study period.
Results
Twenty-six adolescents ages 12–17 M(SD) = 14.70(1.57) completed the study. The sample was half female (50%) and racially diverse with 42% identifying as black or African American, 4% identifying as other, and 54% identifying as White. Family yearly income ranged from $5000 to > $50,000 with the median falling in the $40,000 to $44,999 category. In addition, half of the adolescents were covered by private insurance (50%). See Table 1 for detailed demographic information.
Feasibility and Acceptability
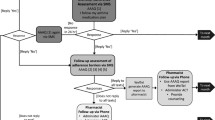
Of the 32 participants approached, two declined, resulting in an enrollment rate of 93.75%. Retention was 83.33% with one participant switching to an incompatible inhaler requiring manual tracking adherence and data that was not included in the adherence analyses. Therefore, of the 30 participants enrolled, 26 (87%) completed Step 1. Step 2 was indicated for 18 (69%) of those participants, 17 (94%) of whom completed Step 2. Those who remained in Step 1 (n = 8) did not differ from those requiring Step 2 (n = 18) on any demographic variables (p’s > .05). Of the 17 participants escalated to Step 2, all 17 (100%) completed all four behavioral sessions (i.e., participation rate of 100%). Overall, the enrollment, retention, and participation rate each surpassed the a priori feasibility criteria. See Fig. 2 for more detail.
This figure depicts participants’ progression through the study with the arrow at the top of the figure displaying the timeline of the study design. 30 participants were enrolled, 26 of whom completed the baseline period of adherence monitoring and received Step 1. Eight participants demonstrated an average adherence higher than 68% and remained in Step 1 for the duration of the study. Of the 18 participants whose average adherence fell below 68% during Step 1, 17 received Step 2, and 1 was lost to follow-up
On average, participants rated their experience in the study between “excellent” and “good” (Macceptability = 3.32) with approximately half (52%) of participants rating their experience in the study as “excellent” and an additional 32% rating their experience as “good.” Only one participant (4%) rated their experience as “poor.” Most participants (64%) indicated satisfaction with the apps. In response to more detailed questions about specific aspects of the intervention, 75% of average ratings were above the acceptability criteria of 3.0. See Fig. 1 for more detail.
Adherence
For the full sample, improvements in adherence from baseline to Step 1 (t = − .47, p = .641, d = .25) were not significant. Adherence did, however, significantly improve from baseline (40%) to the end of the study (58%) when participants received the appropriate intervention based on our stepped-care intervention model with a medium effect size (t = − 2.14, p = .048, d = .52).
For the subsample that received Step 1 only, adherence declined between baseline (69%) and the end of the study (46%) (t = 4.26, p = .013, d = 1.90). Adherence initially increased to 73% for this subset during the first four weeks of Step 1; however, the effect size was small (t = .04, p = .432, d = .35). Adherence for the subsample of those who received Step 2 remained stable from baseline (30%) to Step 1 (33%; t = − .22, p = .826, d = .07), thus reflecting the need for a more intensive Step 2 intervention. Adherence for this subset of patients significantly increased from baseline (30%) to the end of the study (65%, t = − 5.63, p < .001, d = 1.70) with a large effect size (Tables 2, 3).
Disease Control and Lung Function
Overall, a significant and clinically meaningful decrease in CASI (Krouse et al., 2017) was observed from 6.08 to 5.08 with a small effect size (p = .023, d = .48). ACT increased with a small effect size (Table 3). Neither CASI nor ACT were significantly correlated with adherence during the study (p’s > .05). FVC and FEV1 were not significantly correlated with adherence (p’s > .05) and did not significantly change over the course of the study (Table 3).
Discussion
This pilot study demonstrated the feasibility, acceptability, and preliminary efficacy of the first technology-assisted stepped-care intervention to promote adherence in adolescents. Specifically, enrollment, retention, and participation met a priori criteria to establish feasibility and acceptability suggesting that behavioral technology-assisted stepped-care interventions can be delivered to and be engaging for adolescents with asthma. In addition, a significant increase in overall adherence and decrease in asthma composite severity over the course of the intervention suggests a preliminary efficacy of this intervention and that this intervention is worth future research. This stepped-care system also demonstrated promise as a subset of participants required only the initial, least resource-intensive step of the intervention while a larger subset required stepping up to a more intense intervention. Overall this pilot study has provided concrete strengths and weaknesses of technology-assisted stepped-care interventions, support for continued study of this stepped-care adherence intervention, and lessons learned to inform future research in this area.
One of the most substantial strengths of a stepped-care intervention is its “performance-based flexibility,” or that treatment is based on one’s response to previous intervention rather than standardized guidelines (e.g., set number of sessions or modules) (Sobell & Sobell, 2000). This is novel, yet critical for behavior change interventions including adherence promotion interventions due to the ever-changing nature of adherence in response to the wide range of influences throughout childhood, adolescence, and young adulthood (Kenyon et al., 2016; Modi et al., 2012). Future research should examine the use of stepped-care interventions for adherence in larger samples of adolescents with asthma and other chronic conditions. Although stepped-care has not been widely researched in pediatric behavioral health beyond pediatric weight control trials, the feasibility of this intervention combined with emerging research demonstrating the efficacy and cost-effectiveness of stepped-care treatment for adults with depression and anxiety (Bower & Gilbody, 2005; Ho et al., 2016) suggests that stepped-care interventions should be considered for behavioral health concerns beyond adherence.
The digital delivery of this stepped-care intervention is also a significant strength leading to high levels of feasibility and acceptability. Adolescents with adherence difficulties often also struggle with attending medical appointments and the use of technology allowed the intervention to come to them, reducing time and cost burdens. A technology-based intervention system also allows for continued treatment for vulnerable populations during times when face-to-face care may be more challenging to access, such as the current COVID-19 pandemic (Plevinsky et al., 2020) and beyond.
One identified weakness of the implementation of this intervention was the delivery of the app-based content. The acceptability ratings that fell below “good” were in response to the helpfulness of the app-based medication reminders and the appearance and engagement of the spirometry app (e.g., how the app looked, how engaging the app was). Future efforts focused on the design and engagement of the app can be improved by using a user-centered design process with adolescents with asthma to ensure improved design and engagement. Future studies should also investigate user-specific engagement based on demographics, asthma functional severity and control, clinical presentation, need for intervention, and baseline adherence.
Another strength of this study is that the rates of adherence are consistent with adherence rates reported in pediatric asthma research (Bender & Rand, 2004; Drotar & Bonner, 2009; McQuaid & Walders, 2003); therefore, our findings have the potential to be an indicator for a range of youth with asthma. Although this study was powered as a feasibility study and was underpowered for testing efficacy, preliminary efficacy results demonstrated overall pre-post changes in adherence supporting the need for future, larger studies of this technology-assisted stepped-care intervention.
When examining adolescents who only received Step 1 of the intervention, we see that adherence initially increased during the early weeks, but then decreased by the end of the study. This decrease in adherence over time is not surprising as adherence interventions have often demonstrated immediate but not sustained increases in adherence (Pai & McGrady, 2014). This decline in adherence after the initial weeks may also have been due to “reminder fatigue,” the phenomenon that people habituate to digital reminders over time (Muench & Baumel, 2017). Digital alerts alone may not be a sustainable intervention for long-term behavior change (Backman et al., 2017), even for those who demonstrate higher adherence at baseline. Future studies should consider whether to include digital-only interventions and include the systematic adherence assessment over a longer time frame to provide more opportunities to “step-up” care as needed over time.
Specific to the adolescents receiving Step 2 of the intervention, adherence significantly increased by 35%, nearly to the goal of ≥ 68% adherence necessary to reduce morbidity and mortality (Milgrom et al., 1996). Step 2 provided several evidence-based components that likely contributed to this marked increase in adherence such as increased accountability with a live interventionist and tailored problem-solving strategies to reduce barriers to adherence (Zullig et al., 2013). Given that most of the adolescents required stepping up to Step 2 and that there was no improvement with Step 1, future research would benefit from the addition of an intermediate step.
In addition to adherence improvements, participants experienced a clinically meaningful decrease (Krouse et al., 2017) in functional asthma severity (CASI) following the stepped-care intervention. This improvement in asthma functional severity may be due, at least in part, to improved adherence; however, neither asthma control (ACT) nor lung function were significantly improved, and inconsistent relationships were found between adherence and disease variables. Future studies should begin to examine the complex relationship between adherence to medications and clinical outcomes in a larger sample.
Although this study provides evidence for the feasibility, acceptability, and preliminary efficacy of the technology-assisted stepped-care intervention for adolescents with asthma, it is not without limitations. These limitations provide important lessons learned for larger studies of this intervention as well as behavioral stepped-care interventions broadly. First, we encountered several technological difficulties. Participants had difficulty regularly syncing their adherence data during baseline. Although this resulted in missed adherence data, the Bluetooth “in-the-moment” transmission of data allowed us to notice these difficulties and extend the baseline period for some participants. Future studies should implement appropriate system checks to ensure accurate real-time adherence data is being collected. Second, intervention step-ups were dependent on phone contact with participants and study participants were at times difficult to reach by phone resulting in participants being in the study longer than anticipated. Future studies should consider the intensive process of “stepping-up” participants when planning for study personnel, control groups, and data analyses. Third, the pilot nature of the study limited sample size, recruitment pool, and duration of the study. The small size of the sample recruited from a speciality clinic plus the uneven distribution between those who did/did not require Step 2 did not allow us to demonstrate efficacy or detect demographic or disease differences between groups. Given the promising findings of this pilot study, this study should be replicated in a RCT with a larger sample from a variety of settings in order to have the power necessary to test the efficacy of this stepped-care intervention and examine predictors of necessary adherence intervention intensity. Further, this pilot study was designed for each phase to be four weeks long with only one opportunity to step up care. Future studies should examine the benefits of extending the duration of the study periods and provide additional opportunities to step-up care.
Overall, technology-assisted stepped-care is a novel and promising approach to improving adherence to medication and asthma functional severity in adolescents with asthma. Incorporating digital technologies (e.g., electronically monitored adherence, digital reminders, behavioral intervention via telehealth) into stepped-care intervention allows for a systematic and objective measure of adherence and the ability to deliver, adapt, or increase the digital behavioral intervention based on in-the-moment adherence outcomes. Positive feasibility and acceptability data also suggest that adolescents find a technology-assisted stepped-care intervention to increase accessibility and enhance the convenience of receiving asthma management support.
References
Backman, R., Bayliss, S., Moore, D., & Litchfield, I. (2017). Clinical reminder alert fatigue in healthcare: A systematic literature review protocol using qualitative evidence. Systematic Reviews, 6, 255. https://doi.org/10.1186/s13643-017-0627-z
Bell, M. L., Whitehead, A. L., & Julious, S. A. (2018). Guidance for using pilot studies to inform the design of intervention trials with continuous outcomes. Clinical Epidemiology, 10, 153.
Bender, B. G., & Rand, C. (2004). Medication non-adherence and asthma treatment cost. Current Opinion in Allergy and Clinical Immunology, 4(3), 191–195.
Bower, P., & Gilbody, S. (2005). Stepped care in psychological therapies: Access, effectiveness, and efficiency. British Journal of Psychiatry, 186, 11–17.
Cohen, J. (1992). A power primer. Quantitative Methods in Psychology, 112, 155–159.
Dexheimer, J. W., Gu, L., Guo, Y., & Kercsmar, C. (2013). Design of the asthma treat smart system in a pediatric institution. Studies in Health Technology and Informatics, 192, 1004.
Drotar, D., & Bonner, M. S. (2009). Influences on adherence to pediatric asthma treatment: A review of correlates and predictors. Journal of Developmental and Behavioral Pediatrics, 30, 574–582.
duRivage, N., Ross, M., Mayne, S. L., Suh, A., Weng, D., Grundmeier, R. W., & Fiks, A. G. (2017). Asthma control test: Comparing parent proxy with parent and child report for children 6 to 12 years. Clinical Pediatrics, 56, 341–347.
Ho, F. Y., Yeung, W. F., Ng, T. H., & Chan, C. S. (2016). The efficacy and cost-effectiveness of stepped care prevention and treatment for depressive and/or anxiety disorders: A systematic review and meta-analysis. Science and Reports, 6, 29281. https://doi.org/10.1038/srep29281
Jakicic, J. M., Tate, D. F., Lang, W., Davis, K. K., Polzien, K., Rickman, A. D., Erickson, K., Neiberg, R. H., & Finkelstein, E. A. (2012). Effect of a stepped-care intervention appraoch on weight loss in adults: A randomized clinical trial. JAMA, 307, 2614–2626. https://doi.org/10.1001/jama.2012.6866
Jochman, A., Artusio, L., & Jamalzadeh, A. (2017). Electronic monitoring of adherence to inhaled corticosteroids: An essential tool in identifying severe asthma in children. European Respiratory Journal, 50, 1700910.
Kaplan, A., & Price, D. (2020). Treatment adherence in adolescents with asthma. Journal of Asthma and Allergy, 13, 39–49.
Kenyon, C. C., Chang, J., Wynter, S. A., Fowler, J. C., Long, J., & Bryant-Stephens, T. C. (2016). Electronic adherence monitoring in a high-utilizing pediatric asthma cohort: A feasibility study. JMIR Research Protocol, 5, e132. https://doi.org/10.2196/resprot.5362
Krouse, R. Z., Sorkness, C. A., Wildfire, J. J., Calatroni, A., Gruchalla, R., Hershey, G. K. K., Kattan, M., Liu, A. H., Makhija, M., Teach, S. J., West, J. B., Wood, R. A., Zoratti, E. W., & Gergen, P. J. (2017). Minimally important differences and risk levels for the composite asthma severity index. The Journal of Allergy and Clinical Immunology, 139, 1052–1055. https://doi.org/10.1016/j.jaci.2016.08.041
Lewis, M., Bromley, K., Sutton, C. J., McCray, G., Myers, H. L., & Lancaster, G. A. (2021). Determining sample size for progression criteria for pragmatic pilot RCTs: The hypothesis test strikes back! Pilot and Feasibility Studies, 7, 1–14.
Lin, N. Y., Ramsey, R. R., Miller, J. L., McDowell, K. M., Zhang, N., Hommel, K., & Guilbert, T. W. (2020). Telehealth delivery of adherence and medication management system improves outcomes in inner-city children with asthma. Pediatric Pulmonology, 55, 858–865.
McQuaid, E. L., & Walders, N. (2003). Pediatric asthma. In M. C. Roberts (Ed.), Handbook of pediatric psychology. New York: Guilford Press.
Merchant, R., Inamdar, R., Henderson, K., Barrett, M., Su, J. G., Riley, J., Sickle, D. V., & Stempel, D. (2018). Digital health intervention for asthma: Patient-reported value and usability. JMIR mHealth and uHealth, 6, e133. https://doi.org/10.2196/mhealth.7362
Milgrom, H., Bender, B., Ackerson, L., Bowrya, P., Smith, B., & Rand, C. (1996). Noncompliance and treatment failure in children with asthma. Journal of Allergy and Clinical Immunology, 98, 1051–1057.
Miller, M. R., Hankinson, J., Brusasco, V., Burgos, F., Casaburi, R., Coates, A., & Wanger, J. (2005). Standardisation of spirometry. European Respiratory Journal, 26, 319–338. https://doi.org/10.1183/09031936.05.00034805
Modi, A. C., Pai, A. L., Hommel, K. A., Hood, K. K., Cortina, S., Hilliard, M. E., & Drotar, D. (2012). Pediatric self-management: A framework for research, practice, and policy. Pediatrics, 129, e473-485. https://doi.org/10.1542/peds.2011-1635
Morton, R. W., Everard, M. L., & Elphick, H. E. (2014). Adherence in childhood asthma: The elephant in the room. Archives of Disease in Childhood, 99, 949–953.
Muench, F., & Baumel, A. (2017). More than a text message: Dismantling digital triggers to curate behavior change in patient-centered health interventions. Journal of Medical Internet Research, 19, e147. https://doi.org/10.2196/jmir.7463
National Asthma Education and Prevention Program. (2007). Expert panel report 3 (EPR-3): Guidlines for the diagnosis and management of asthma-summary report. Journal of Allergy and Clinical Immunology, 120, S94–S138.
Nicholas, J., Ringland, K. E., Graham, A. K., Knapp, A. A., Lattie, E. G., Kwasny, M. J., & Mohr, D. C. (2019). Stepping Up: Predictors of “Stepping” within an iCBT stepped-care intervention for depression. International Journal of Environmental Research and Public Health. https://doi.org/10.3390/ijerph16234689
Pai, A. L., & McGrady, M. (2014). Systematic review and meta-analysis of psychological interventions to promote treatment adherence in children, adolescents, and young adults with chronic illness. Journal of Pediatric Psychology, 39, 918–931.
Perry, R., Braileanu, G., Palmer, T., & Stevens, P. (2019). The economic burden of pediatric asthma in the United States: Literature review of current evidence. PharmacoEconomics, 37, 155–167. https://doi.org/10.1007/s40273-018-0726-2
Plevinsky, J. M., Young, M. A., Carmody, J. K., Durkin, L. K., Gamwell, K. L., Klages, K. L., Ghosh, S., & Hommel, K. A. (2020). The impact of COVID-19 on pediatric adherence and self-management. Journal of Pediatric Psychology. https://doi.org/10.1093/jpepsy/jsaa079
Prevention, C. F. D. C. A. (2016). National health interview survey.
Ramsey, R. R., Carmody, J. K., Holbein, C. E., Guilbert, T. W., & Hommel, K. A. (2018). Examination of the uses, needs, and preferences for health technology use in adolescents with asthma. Journal of Asthma. https://doi.org/10.1080/02770903.2018.1514048
Ramsey, R. R., Plevinsky, J. M., Kollin, S. R., Gibler, R. C., Guilbert, T. W., & Hommel, K. A. (2020). Systematic review of digital interventions for pediatric asthma management. Journal of Allergy and Clinical Immunology: In Practice, 8, 1284–1293.
Sobell, M. B., & Sobell, L. C. (2000). Stepped care as a heuristic approach to the treatment of alcohol problems. Journal of Counseling and Clinical Psychology, 68, 573–579.
Sullivan, P. W., Ghushchyan, V., Navaratnam, P., Friedman, H. S., Kavati, A., Ortiz, B., & Lanier, B. (2017). The national cost of asthma among school-aged children in the United States. Annals of Allergy, Asthma & Immunology, 119, 246-252.e241. https://doi.org/10.1016/j.anai.2017.07.002
Wang, X., Dockery, D. W., Wypij, D., Fay, M. E., & Ferris, B. G., Jr. (1993). Pulmonary function between 6 and 18 years of age. Pediatric Pulmonology, 15, 75–88. https://doi.org/10.1002/ppul.1950150204
Wildfire, J. J., Gergen, P. J., Sorkness, C. A., Mitchell, H. E., Calatroni, A., Kattan, M., & Wood, R. A. (2012). Development and validation of the composite asthma severity index—an outcome measure for use in children and adolescents. Journal of Allergy and Clinical Immunology, 129, 694–701.
Zullig, L. L., Peterson, E. D., & Bosworth, H. B. (2013). Ingredients of successful interventions to improve medication adherence. JAMA, 310, 2611–2612. https://doi.org/10.1001/jama.2013.282818
Acknowledgements
This work was supported by the United States National Institutes of Health, including a career development award from the National Heart, Lung, and Blood Institute (K23 HL139992) and a training grant from the National Institute of Child Health and Human Development (T32 HD068223).
Funding
Funding was provided by National Heart, Lung, and Blood Institute (K23HL139992) [National Institute of child health and human development (T32 HD068223)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Guilbert reports personal fees from American Board of Pediatrics; Pediatric Pulmonary Subboard, personal fees from GSK, personal fees from TEVA, personal fees from Novartis, grants from NIH, grants and personal fees from Sanofi/Regeneron, grants and personal fees from Astra-Zeneca, royalties from UpToDate. All other authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramsey, R.R., Plevinsky, J.M., Guilbert, T.W. et al. Technology-Assisted Stepped-Care to Promote Adherence in Adolescents with Asthma: A Pilot Study. J Clin Psychol Med Settings 30, 415–424 (2023). https://doi.org/10.1007/s10880-022-09905-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10880-022-09905-5