Abstract
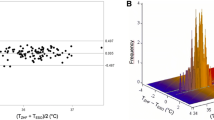
General anesthesia impairs thermoregulation and contributes to perioperative hypothermia; core body temperature monitoring is recommended during surgical procedures lasting > 30 min. Zero-heat-flux core body temperature measurement systems enable continuous non-invasive perioperative monitoring. During a previous trial evaluating the benefits of preoperative forced-air warming, intraoperative temperatures were measured with both a zero-heat-flux sensor and a standard naso-/oropharyngeal temperature probe. The aim of this secondary analysis is to evaluate their agreement. ASA I–III patients, scheduled for elective, non-cardiac surgery under general anesthesia, were enrolled. A zero-heat-flux sensor was placed on the participant’s forehead preoperatively. Following induction of anesthesia, a “clinical” temperature probe was placed in the nasopharynx or oropharynx at the anesthesiologist’s discretion. Temperature measurements from both sensors were recorded every 10 s. Agreement was analyzed using the Bland–Altman method, corrected for repeated measurements, and Lin’s concordance correlation coefficient, and compared with existing studies. Data were collected in 194 patients with a median (interquartile range) age of 60 (49–69) years, during surgical procedures lasting 120 (89–185) min. The zero-heat-flux measurements had a mean bias of − 0.05 °C (zero-heat-flux lower) with 95% limits of agreement within − 0.68 to + 0.58 °C. Lin’s concordance correlation coefficient was 0.823. The zero-heat-flux sensor demonstrated moderate agreement with the naso-/oropharyngeal temperature probe, which was not fully within the generally accepted ± 0.5 °C limit. This is consistent with previous studies. The zero-heat-flux system offers clinical utility for non-invasive and continuous core body temperature monitoring throughout the perioperative period using a single sensor.




Similar content being viewed by others
References
Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318–38.
Dobson G, Chong M, Chow L, Flexman A, Kurrek M, Laflamme C, et al. Guidelines to the practice of anesthesia—revised edition 2018. Can J Anesth (Can d’Anesthésie). 2018;65:76–104.
Eshraghi Y, Nasr V, Parra-Sanchez I, Van Duren A, Botham M, Santoscoy T, et al. An evaluation of a zero-heat-flux cutaneous thermometer in cardiac surgical patients. Anesth Analg. 2014;119:543–9.
Pawley MDM, Martinsen P, Mitchell SJ, Cheeseman JF, Merry AF, Willcox T, et al. Brachial arterial temperature as an indicator of core temperature: proof of concept and potential applications. J Extra Corpor Technol. 2013;45:86–93.
Selvaraj V, Gnanaprakasam PV. Evaluation of skin temperature over carotid artery for temperature monitoring in comparison to nasopharyngeal temperature in adults under general anesthesia. Anesth Essays Res. 2016;10:291–6.
Evron S, Weissman A, Toivis V, Shahaf DB, You J, Sessler DI, et al. Evaluation of the temple touch pro, a novel noninvasive core-temperature monitoring system. Anesth Analg. 2017;125:103–9.
Fox RH, Solman AJ, Isaacs R, Fry AJ, MacDonald IC. A new method for monitoring deep body temperature from the skin surface. Clin Sci. 1973;44:81–6.
Lees DE, Kim YD, Macnamara TE. Noninvasive determination of core temperature during anesthesia. South Med J. 1980;73:1322–4.
Sastre JA, Pascual MJ, López T. Evaluation of the novel non-invasive zero-heat-flux Tcore™ thermometer in cardiac surgical patients. J Clin Monit Comput. 2019;33:165–72.
Mäkinen M-T, Pesonen A, Jousela I, Päivärinta J, Poikajärvi S, Albäck A, et al. Novel cardiac zero-heat-flux deep body temperature measurement in lower extremity vascular and surgery. J Cardiothorac Vasc Anesth. 2016;30:973–8.
Iden T, Horn E-P, Bein B, Böhm R, Beese J, Höcker J. Intraoperative temperature monitoring with zero heat flux technology (3M SpotOn sensor) in comparison with sublingual and nasopharyngeal temperature: an observational study. Eur J Anaesthesiol. 2015;32:387–91.
Kollmann Camaiora A, Brogly N, Alsina E, de Celis I, Huercio I, Gilsanz F. Validation of the Zero-Heat-Flux thermometer (SpotOn®) in major gynecological surgery to monitor intraoperative core temperature: a comparative study with esophageal core temperature. Minerva Anestesiol. 2019;85:351–7.
Boisson M, Alaux A, Kerforne T, Mimoz O, Debaene B, Dahyot-Fizelier C, et al. Intra-operative cutaneous temperature monitoring with zero-heat-flux technique (3M SpotOn) in comparison with oesophageal and arterial temperature: a prospective observational study. Eur J Anaesthesiol. 2018;35:825–30.
Pesonen E, Silvasti-Lundell M, Niemi TT, Kivisaari R, Hernesniemi J, Mäkinen M-T. The focus of temperature monitoring with zero-heat-flux technology (3M Bair-Hugger): a clinical study with patients undergoing craniotomy. J Clin Monit Comput. 2019;33:917–923. https://doi.org/10.1007/s10877-018-0227-z.
Jack JM, Ellicott H, Jones CI, Bremner SA, Densham I, Harper CM. Determining the accuracy of zero-flux and ingestible thermometers in the peri-operative setting. J Clin Monit Comput. 2019. https://doi.org/10.1007/s10877-019-00252-9.
Carvalho H, Najafi N, Poelaert J. Intra-operative temperature monitoring with cutaneous zero-heat- flux-thermometry in comparison with oesophageal temperature: a prospective study in the paediatric population. Paediatr Anaesth. 2019;29:865–871. https://doi.org/10.1111/pan.13653.
3M SpotOn™ temperature monitoring system model 370, p. 22. 2013 [cited 2019 Oct 28]. https://multimedia.3m.com/mws/media/879803O.
Lau A, Lowlaavar N, Cooke EM, West N, German A, Morse DJ, et al. Effect of preoperative warming on intraoperative hypothermia: a randomized-controlled trial. Can J Anesth. 2018;65:1029–40.
Institute of Social and Preventive Medicine University of Bern. STROBE Statement: Strenghtening the reporting of observational studies in epidemilogy. 2009 [cited 2019 Apr 8]. https://www.strobe-statement.org.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–82.
Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68.
Schell-Chaple HM, Liu KD, Matthay MA, Puntillo KA. Rectal and bladder temperatures vs forehead core temperatures measured with SpotOn monitoring system. Am J Crit Care. 2018;27:43–50.
McBride G. A proposal for strength-of-agreement criteria for Lin’s concordance correlation coefficient. Natl Inst Water Atmos Res Client Rep Minist Health, pp. 1–10. 2005 [cited 2019 Jan 9]. https://www.medcalc.org/download/pdf/McBride2005.pdf.
Kimberger O, Thell R, Schuh M, Koch J, Sessler DI, Kurz A. Accuracy and precision of a novel non-invasive core thermometer. Br J Anaesth. 2009;103:226–31.
Zeiner A, Klewer J, Sterz F, Haugk M, Krizanac D, Testori C, et al. Non-invasive continuous cerebral temperature monitoring in patients treated with mild therapeutic hypothermia: an observational pilot study. Resuscitation. 2010;81:861–6.
Sinha PK, Kaushik S, Neema PK. Massive epistaxis after nasopharyngeal temperature probe insertion after cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:123–4.
Wang M, Singh A, Qureshi H, Leone A, Mascha EJ, Sessler DI. Optimal depth for nasopharyngeal temperature probe positioning. Anesth Analg. 2016;122:1434–8.
Cork RC, Vaughan RW, Humphrey LS. Precision and accuracy of intraoperative temperature monitoring. Anesth Analg. 1983;62:211–4.
Roth JV, Braitman LE. Nasal temperature can be used as a reliable surrogate measure of core temperature. J Clin Monit Comput. 2008;22:309–14.
Lim H, Kim B, Kim D-C, Lee S-K, Ko S. A comparison of the temperature difference according to the placement of a nasopharyngeal temperature probe. Korean J Anesthesiol. 2016;69:357–61.
Lee J, Lim H, Son K-G, Ko S. Optimal nasopharyngeal temperature probe placement. Anesth Analg. 2014;119:875–9.
van Zundert A, Wyssusek K, Vivian V. Verification of nasopharyngeal temperature probes-they are not always where you think they are! Anesth Analg. 2016;123:1338–9.
Taylor NAS, Tipton MJ, Kenny GP. Considerations for the measurement of core, skin and mean body temperatures. J Therm Biol. 2014;46:72–101.
Acknowledgements
The authors would like to thank all the participating patients and anesthesiologists, the surgical and nursing teams in the operating room, as well as Aaron Lau, Nasim Lowlaavar and Alexandra German for their help with the data collection.
Funding
3M Canada sponsored the original randomized controlled trial, from which the data for this secondary was extracted, and provided the 3M Bair Hugger™ temperature monitoring systems and sensors.
Author information
Authors and Affiliations
Contributions
MG, EC, NW and RNM designed the study and obtained ethical approval to conduct the research. EC performed the data collection. DM and MG analyzed the data. NW and MG interpreted the findings and drafted the manuscript. All authors critically reviewed the manuscript, and read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
Dan Morse is a salaried employee of 3M. The other authors have no conflicts of interest.
Research involving human participants
This study reports secondary analysis of data obtained in a previous trial that was conducted with research ethics board approval (Fraser Health Research Ethics Board, FHREB 2014-02).
Informed consent
Written informed consent was obtained from all individual participants in the original study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
West, N., Cooke, E., Morse, D. et al. Zero-heat-flux core temperature monitoring system: an observational secondary analysis to evaluate agreement with naso-/oropharyngeal probe during anesthesia. J Clin Monit Comput 34, 1121–1129 (2020). https://doi.org/10.1007/s10877-019-00411-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00411-y