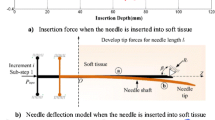
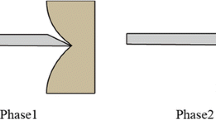
Abstract
This work aims to introduce a new needle insertion simulation to predict the deflection of a bevel-tip needle inside soft tissue. The development of such a model, which predicts the steering behavior of the needle during needle-tissue interactions, could improve the performance of many percutaneous needle-based procedures such as brachytherapy and thermal ablation, by means of the virtual path planning and training systems of the needle toward the target and thus reducing possible incidents of complications in clinical practices. The Arbitrary–Lagrangian–Eulerian (ALE) formulation in LS-DYNA software was used to model the solid–fluid interactions between the needle and tissue. Since both large deformation and fracture of the continuum need to be considered in this model, applying ALE method for fluid analysis was considered a suitable approach. A 150 mm long needle was used to bend within the tissue due to the interacting forces on its asymmetric bevel tip. Three experimental cases of needle steering in a soft phantom were performed to validate the simulation. An error measurement of less than 10 % was found between the predicted deflection by the simulations and the one observed in experiments, validating our approach with reasonable accuracy. The effect of the needle diameter and its bevel tip angle on the final shape of the needle was investigated using this model. To maneuver around the anatomical obstacles of the human body and reach the target location, thin sharp needles are recommended, as they would create a smaller radius of curvature. The insertion model presented in this work is intended to be used as a base structure for path planning and training purposes for future studies.








Similar content being viewed by others
References
Podder TK, Dicker AP, Hutapea P, Yu Y. A novel curvilinear approach for prostate seed implantation. J Med Phys. 2012;39:1887–92. doi:10.1118/1.3694110.
Engh JA, Podnar G, Kondziolka D, Riviere CN. Toward effective needle steering in brain tissue. In: IEEE EMBS 28th annual international conference, New York City, NY; 2006, p. 559–62
Crouch JR, Schneider CM, Wainer J, Okamura AM. A velocity-dependent model for needle insertion in soft tissue. Med Image Comput Comput Assist Interv. 2005;8:624–32. doi:10.1007/11566489_77.
Reed BKB, Majewicz A, Kallem V, Alterovitz R, Goldberg K, Cowan NJ, Okamura AM. Robot-assisted needle steering. IEEE Robot Autom Mag. 2011;18:35–46. doi:10.1109/MRA.2011.942997.
Webster RJ, Romano JM, Member S, Cowan NJ. Mechanics of precurved-tube continuum robots. Robot IEEE Trans. 2009;25:67–78. doi:10.1109/TRO.2008.2006868.
Wedlick TR, Okamura AM. Characterization of pre-curved needles for steering in tissue. Annu Int Conf IEEE Eng Med Biol Soc. 2009;. doi:10.1109/IEMBS.2009.5333407.
Swaney PJ, Burgner J, Gilbert HB, WebsterIII RJ. A flexure-based steerable needle: high curvature with reduced tissue damage. IEEE Trans Biomed Eng. 2013;60:906–9. doi:10.1109/TBME.2012.2230001.
Datla NV, Konh B, Koo J, Daniel WC, Yu Y, Dicker AP, Podder TK, Darvish K, Hutapea P. Polyacrylamide phantom for self-actuating needle–tissue interaction studies. Med Eng Phys. 2014;36:140–5. doi:10.1016/j.medengphy.2013.07.004.
Datla NV, Konh B, Honarvar M, Podder TK, Dicker AP, Yu Y, Hutapea P. A model to predict deflection of bevel-tipped active needle advancing in soft tissue. Med Eng Phys. 2013;36:258–93. doi:10.1016/j.medengphy.2013.11.006.
Konh B, Datla NV, Hutapea P. Feasibility of SMA wire actuation for an active steerable cannula. J Med Device. 2014;10(1115/1):4029557. doi:10.1115/1.4029557.
Honarvar M, Datla NV, Konh B, Podder TK, Dicker AP, Yu Y, Hutapea P. Study of unrecovered strain and critical stresses in one-way shape memory Nitinol. Mater Eng Perform. 2014;23:2885–93. doi:10.1007/s11665-014-1077-6.
Konh B, Honarvar M, Hutapea P. Design optimization study of a shape memory alloy active needle for biomedical applications. J Med Eng Phys. 2015;37:469–77. doi:10.1016/j.medengphy.2015.02.013.
Ryu SC, Quek ZF, Koh JS, Renaud P, Black RJ, Moslehi B, Daniel BL, Cho KJ, Cutkosky MR. Design of an optically controlled MR-compatible active needle. IEEE Trans Robot. 2015;31:1–11. doi:10.1109/TRO.2014.2367351.
Ayvali E, Desai JP. Pulse width modulation-based temperature tracking for feedback control of a shape memory alloy actuator. J Intell Mater Syst Struct. 2014;25:720–30. doi:10.1177/1045389X13502576.
van de Berg NJ, van Gerwen DJ, Dankelman J, van den Dobbelsteen JJ. Design choices in needle steering—a review. Mechatron IEEE/ASME Trans. 2015;20:2172–83. doi:10.1109/TMECH.2014.2365999.
Abolhassani N, Patel R, Moallem M. Needle insertion into soft tissue: a survey. Med Eng Phys. 2007;29:413–31. doi:10.1016/j.medengphy.2006.07.003.
van Gerwen DJ, Dankelman J, van den Dobbelsteen JJ. Needle–tissue interaction forces—a survey of experimental data. Med Eng Phys. 2012;34:665–80. doi:10.1016/j.medengphy.2012.04.007.
Alterovitz R, Goldhere K, Berkeley UC. Needle insertion and radioactive seed implantation in human tissues: simulation and sensitivity analysis’. In: Robot Autom 2003 Proceedings ICRA’03 IEEE Int Conf 2003; 2:1793–9. doi:10.1109/ROBOT.2003.1241854.
DiMaio SP, Salcudean SE. Interactive simulation of needle insertion models. IEEE Trans Biomed Eng. 2005;52:1167–79. doi:10.1109/TBME.2005.847548.
Nienhuys H-W, van der Stappen AF. A computational technique for interactive needle insertions in 3D nonlinear material. In: Robot autom 2004 proceedings ICRA’04 2004 IEEE int conf 2004; 2:2061–67. doi:10.1109/ROBOT.2004.1308127.
Misra S, Reed KB, Schafer BW, Ramesh KT, Okamura AM. Mechanics of flexible needles robotically steered through soft tissue. Int J Rob Res. 2010;29:1640–60. doi:10.1177/0278364910369714.
Okamura AM, Simone C, O’Leary M. Force modeling for needle insertion into soft tissue. Biomed Eng IEEE Trans. 2004;51:1707–16. doi:10.1109/TBME.2004.831542.
Roesthuis RJ, Abayazid M, Misra S. Mechanics-based model for predicting in-plane needle deflection with multiple bends. In: 2012 4th IEEE RAS EMBS int conf biomed robot biomechatronics 2004; p. 69–74. doi:10.1109/BioRob.2012.6290829.
Webster RJ. Nonholonomic modeling of needle steering. Int J Rob Res. 2006;25:509–25. doi:10.1177/0278364906065388.
Kataoka H, Washio T, Audette M, Mizuhara K. A model for relations between needle deflection, force, and thickness on needle penetration. Med image Comput Comput Interv. 2001;. doi:10.1007/3-540-45468-3_115.
Abolhassani N, Patel R. Deflection of a flexible needle during insertion into soft tissue. In: Eng Med Biol Soc 2006 EMBS’06 28th Annu Int Conf IEEE 2006; p. 3858–61.
Podder TK, Clark D, Sherman J, Fuller D, Messing E, Rubens D, Strang J, Brasacchio L, Liao WSN, Yu Y. In Vivo motion and force measurement of surgical needle intervention during prostate brachytherapy. J Med Phys. 2006;33:2915–22.
Majewicz A, Wedlick TR, Reed KB, Okamura AM. Evaluation of robotic needle steering in ex vivo tissue. IEEE Int Conf Robot Autom. 2010;2010:2068–73. doi:10.1109/ROBOT.2010.5509873.
Oldfield M, Dini D, Giordano G, Rodriguez y Baena F. Detailed finite element modelling of deep needle insertions into a soft tissue phantom using a cohesive approach. Comput Methods Biomech Biomed Eng. 2013;16:530–43. doi:10.1080/10255842.2011.628448.
Kataoka H, Noda S, Yokota H, Takagi S, Himeno R, Okazawa S. Simulations of needle insertion by using a Eulerian hydrocode FEM and the experimental validations. Med Image Comput Comput Interv. 2008;. doi:10.1007/978-3-540-85990-1-67.
Yamaguchi S, Tsutsui K, Satake K, Morikawa S, Shirai Y, Tanaka HT. Dynamic analysis of a needle insertion for soft materials: arbitrary Lagrangian–Eulerian-based three-dimensional finite element analysis. Comput Biol Med. 2014;53:42–7. doi:10.1016/j.compbiomed.2014.07.012.
Hirt CW, Amsden AA, Cook JL. An arbitrary Lagrangian–Eulerian computing method for all flow speeds. J Comput Phys. 1974;14:227–53. doi:10.1016/0021-9991(74)90051-5.
LSTC. LS-DYNA Theory Manual Version 970. r:6030. Livernire Software Technology Corporation (LSTC), Livermore, CA.
Ahn BM, Kim J, Ian L, Rha KH, Kim HJ. Mechanical property characterization of prostate cancer using a minimally motorized indenter in an ex vivo indentation experiment. Urology. 2010;76:1007–11. doi:10.1016/j.urology.2010.02.025.
Shergold OA, Fleck NA. Experimental investigation into the deep penetration of soft solids by sharp and blunt punches, with application to the piercing of skin. J Biomech Eng. 2005;127:838–48. doi:10.1115/1.1992528.
Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–83. doi:10.1557/JMR.1992.1564.
Hertz HR. On the contact of rigid elastic solids and on hardness. New York, NY: McMillan; 1882.
Acknowledgments
This study was funded by the Department of Defense CDMRP Prostate Cancer Research Program (Grant # W81XWH-11-1-0398).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Konh, B., Honarvar, M., Darvish, K. et al. Simulation and experimental studies in needle–tissue interactions. J Clin Monit Comput 31, 861–872 (2017). https://doi.org/10.1007/s10877-016-9909-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9909-6