Abstract
Respiratory problems occur more frequently in patients who undergo open heart surgery. Intraoperative and postoperative ventilation strategies can prevent these complications and reduce mortality. We hypothesized that PCV would have better effects on gas exchange, lung mechanics and hemodynamics compared to VCV in CABG surgery. Our primary outcome was to compare the PaO2/FiO2 ratio. Patients were randomized into two groups, (VCV, PCV) consisting of 30 individuals each. Two patients were excluded from the study. I/E ratio was adjusted to 1:2 and, RR:10/min fresh air gas flow was set at 3L/min in all patients. In the VCV group TV was set at 8 mL/kg of the predicted body weight. In the PCV group, peak inspiratory pressure was adjusted to the same tidal volume with the VCV group. PaO2/FiO2 was found to be higher with PCV at the end of the surgery. Time to extubation and ICU length of stay was shorter with PCV. Ppeak was similar in both groups. Pplateau was lower and Pmean was higher at the and of the surgery with PCV compared to VCV. The hemodynamic effects of both ventilation modes were found to be similar. PVC may be preferable to VCV in patients who undergo open heart surgery. However, it would be convenient if our findings are supported by similar studies.
Similar content being viewed by others
1 Introduction
Postoperative pulmonary complications (POPC) following cardiac surgery occur more frequently in patients than in those who undergo other surgical procedures and compared to cardiac causes, they increase mortality more often [1, 2]. Atelectasis, increase in shunt, changes occurring in pulmonary and chest wall mechanics, alterations in capillary bed and pulmonary parenchyma due to left ventricle dysfunction, surgery, anesthesia, cardiopulmonary bypass (CPB) and inflammatory response secondary to mechanical ventilation are among the causes of POPC [3, 4]. Perioperative appropriate ventilatory strategies may minimize pulmonary functions changes and reduce incidence of POPC [5]. There is no consensus in the literature regarding the best ventilatory modality to be used [6]. Pressure controlled ventilation (PCV) appears to be associated with earlier respiratory system mechanics’ recovery as compared with volume controlled ventilation (VCV) [7]. In a review by Ferrando et al. [8], lung protective ventilation with less than 10 mL/kg tidal volume (TV) was recommended as an intraoperative lung protective ventilation strategy in open heart surgery and no distinction was made as to being pressure/volume controlled.
VCV is a mechanical ventilation mode frequently preferred during anesthesia. VCV may lead to decrease compliance of the lungs and follow by increasing peak airway pressure (Ppeak) and plateau pressure (Pplateau) and lung damage associated with ventilator can occur. PCV may offer particular advantages in certain circumstances in which variable flow rates are preferred or when pressure and volume limitation is required [9]. Maximum airway pressure to the lungs is restricted by PCV, while TV and minute volume (MV) may not be guaranteed [10]. Incidence of acute respiratory distress syndrome (ARDS) following coronary artery bypass graft surgery (CABG) ranges between 0.4 and 2.0 %, whereas its mortality is considerably high [11, 12]. It is reported that the PaO2/FiO2 ratio following open heart surgery is the most important marker in detecting the prognosis during the early period [13]. In recent years PCV has been considered as ventilatory strategy in patients with ARDS [7, 14–16]. We hypothesized that PCV would have better effects on gas exchange, lung mechanics and hemodynamics compared to VCV in CABG surgery. Our primary outcome was to compare the PaO2/FiO2 ratio. Our secondary goals were to compare the effects on time to extubation, intensive care unit (ICU) length of stay, hemodynamic and respiratory mechanics.
2 Materials and methods
After obtaining local ethical committee approval and informed consent, 60 patients who would undergo elective CABG for the first time were included in the study. Patients with previous major obstructive or restrictive pulmonary disease (defined as, 70 % of predicted values for pulmonary function test variables of volume and flow), pulmonary hypertension (pulmonary artery pressure >35 mmHg, based on preoperative transthoracic echocardiography), poor ventricular function (ejection fraction <35 %), congestive heart failure, renal failure (serum creatinine >1.8 mg/dL), morbid obesity [body mass index (BMI) >35 kg/m2], anemia [haemoglobin (Hb) <10 g/dL], and history of smoking until 2 months ago were excluded from the study. Patients who underwent re-exploration for any reason were not included in the study. Patients were premedicated with intravenous (iv) midazolam before being transferred to the operating room. 5 L/min oxygen was given via face mask, heart rate (HR) was monitored with 5-channel electrocardiography, and standard peripheral oxygen saturation (SpO2) and noninvasive blood pressure monitorization were performed. After local anesthetic and iv fentanly (1–2 μg/kg) administration a specific thermistor-tipped catheter (VolumeView™ catheter, 5 Fr, 20 cm, Edwards, Lifesciences, Irvine, USA) was placed into the right femoral artery. After anesthesia induction carried out with 0.05–0.1 mg/kg midazolam, 5–10 μg/kg fentanyl, 0.1 mg/kg rocuronium and 2–3 mg/kg thiopental, male patients were intubated with an 8.0 internal diameter (ID) endotracheal tube (ETT), and female patients were intubated with a 7.5 ID ETT. A central venous pressure (CVP) catheter was placed preferably into the right internal jugular vein. Arterial and venous catheters were connected to the EV1000 cardiac output (CO) monitor (Edwards Lifesciences, Irvine, USA). Bolus administration of 15 cc saline, kept at below 15 °C, was performed in 2 s through the CVP catheters of the patient and this procedure was repeated 3 times to ensure the calibration of the device. Maintenance of anesthesia was provided with 40 % oxygen and 60 % air mixture, desfluran (0.5–1 MAC) inhalation and remifentanyl infusion (0.5–1 μg/kg/min). Intraoperative additional analgesia was provided with iv bolus fentanyl. CABG was performed through a median sternotomy with heparinization under CPB using aortic and two-stage atriovenous cannulation. CPB was initiated using a membrane oxygenator with a non-pulsatile flow rate of 2.2–2.4 L/min/m2 and a mean arterial pressure (MAP) of 50–80 mmHg. Moderate systemic hypothermia around 30 °C was induced during CPB. Arterial oxygen tension (PaO2) was kept at 90–150 mmHg, arterial carbon dioxide tension (PaCO2) at 35–45 mmHg and venous oxygen saturation (SvO2) >70 % in the CPB period. Myocardial protection was achieved with antegrade hyperkalemic blood cardioplegia. The lungs were not ventilated during the CPB and connected to the bain circuit with a basal oxygen flow of 200 mL/min. Before discontinuation of CPB, lungs were manually inflated until visible atelectasis disappeared. CPB was terminated with the same ventilator setting as before CPB. Balanced electrolyte solution (Isolyte S) was primarily preferred for fluid replacement and in case of stroke volume variability (SVV) >13 %, stroke volume (SV) was tried to be optimized by giving 250–500 mL colloid. Vasopressor and inotropic agent need was determined according to stroke volume index (SVI), systemic vascular resistance index (SVRI) and MAP values so that cardiac index (CI) = 2.5–4.2 L/min/m2 is maintained. Erythrocyte suspension was administered in case of initial values of SvO2 <70 %, haematocrit (Htc) <24 % and near-infrared spectroscopy <20 %.
2.1 Study protocol
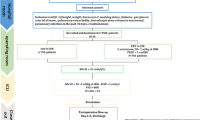
The patients were randomized into two groups as the volume-controlled (Group VCV) and pressure-controlled (Group PCV) groups, consisting of 30 individuals each. Randomization was carried out using the sequentially numbered opaque sealed envelope technique (SNOSE) [17]. All patients were ventilated with same anesthesia equipment (Draeger, Primus, Draeger Medical AG&Co, Germany). The TV was set based on the predicted body weight. [i.e. 50 + 0.91_(height in cm—152.4) for men and 45.5 + 0.91_(height in cm—152.4) for women] [18]. TV was initially set at 8 mL/kg of predicted weight in the VCV group, the Inspiration/Expiration (I/E) ratio was adjusted to 1:2, and plateau time was set at 20 % of inspiratory time. Mechanical ventilation was provided with square-wave flow in the VCV. In the PCV mode, a drop to zero inspiratory flow was checked on the flow-time curve to maximize the TV generated for a given level of inspiratory pressure and to allow a comparison of Pplateau between the two modes [19]. The peak inspiratory pressure (PIP) was initiated with 15 cmH2O and was set at 8 mL/kg TV for the patients in the PCV group. No external positive end expiratory pressure was applied throughout the entire study. The respiratory rate (RR) was initially set at 10/min in both groups. The end-tidal carbon dioxide (EtCO2) values was maintained between 30 and 35 mmHg and Ppeak was kept as low as possible with an upper limit of Ppeak 35 cmH2O, according to a distinct algorithm for each ventilation mode. Oxygen concentration was increased when SpO2 dropped below 97 %. (The study protocol is summarized in Fig. 1). Expired TV, RR, Tinspiratory/Ttotal (Ti/Ttot), FiO2, systolic and diastolic arterial pressures (SAP, DAP) and end-tidal desflurane concentration were assessed with data obtained with 15 min of intervals. Data obtained from arterial blood gas (ABG) (pH, PaO2, PaCO2, lactate, Hb, Htc), PaO2/FiO2 ratio, CI, SVI, SVRI, CVP, Ppeak, Pplateau, mean airway pressure (Pmean) and static compliance (Cstat) were recorded three times at 15 min prior to CPB (T1), 15 min following CPB (T2) and the end of the surgery (T3).
2.2 Statistical analyses
In our pilot study, end-surgery arterial blood gas analyses had revealed that the PaO2/FiO2 ratio was 300 ± 55 mmHg in open heart surgery patients who were ventilated with VCV. Predicting that this rate would increase by 10 % with PCV, we calculated the number of patients for each group as 27. Considering the possible patients who could be excluded from the study, we determined the number of patients for each group as 30. All statistical analyses were performed using IBM SPSS for Windows version 20.0 (SPSS, Chicago, IL, USA). Kolmogorov–Smirnov tests were used to test the normality of the data distribution. Continuous variables were expressed as mean ± standard deviation, median (between the 25th and 75th percentiles), and categorical variables were expressed as counts (percentages). Comparisons of continuous variables between the groups were performed using the Student’s t test and Mann–Whitney U Test. Comparisons of continuous paired variables were performed using the Two Way Analysis of Variance test, Friedman Two Way Analysis of Variance test and Dunn’s Multiple Comparison Test. Comparisons of categorical variables between the groups were performed using the Yates’ Chi-square test and Fisher’s Chi-square test. A two-sided p value of <0.05 was considered statistically significant.
3 Results
One patient was excluded due to mortality at postoperative 72nd hour in the PCV group and one case was excluded in the VCV group because the patient was reexplored; therefore, data of 29 patients were taken into evaluation from each group (58 patients in total Fig. 2). Preoperative characteristics were similar in both groups (Table 1). Intraoperative features were also similar. Normal course of EtCO2 could be provided with similar RR and MV in both groups. Both ventilation techniques affected the SAP, DAP and HR similarly. Time to extubation was significantly shorter in the PCV group, (p < 0.001), (Table 2). PaO2 at the end of the surgery was statistically significantly higher in the PCV group, (p = 0.001). Other statistically significant changes in ABG parameters were not considered clinically significant since the data were within physiologic limits (Table 3). The PaO2/FiO2 ratio was significantly higher at the end of the surgery in the PCV group, (p = 0.019), (Table 4). Cardiac contractility, venous return and systemic resistance were similarly affected by both ventilation modes (Table 5). The effect of both ventilation modes on Ppeak was similar. Pplateau was significantly lower both after CPB and at the end of surgery in the PCV group as compared to the VCV group, (p = 0.01; p = 0.004). Pmean at the end of the surgery was higher in the PCV group, (p = 0.02). Cstat was higher at all times during PCV compared to VCV, (p = 0.03; p = 0.005; p < 0.001). However, it decreased significantly at the end of the surgery compared to the pre-CPB values in both groups (VCV < 0.001; PCV = 0.01), (Table 6).
4 Discussion
According to the results of our study, although the PaO2/FiO2 ratio was similar with both ventilation modes during the intraoperative period, it was found to be higher with PCV at the end of the surgery. This feature can outmaneuver PCV by improving PaO2/FiO2, which is reported to be crucial in detecting mortality during the early postoperative period.
The two differences between VCV and PCV are the flow pattern and the chosen target. VCV mode utilizes a constant flow (to deliver a target TV) and thus insures a satisfactory MV, PCV uses a decelerating flow which reaches the highest possible value at the beginning of inspiration, while having a preset pressure limitation but no minimum TV. Flow diminishes throughout inspiration according to the pressure target, and the resulting TV depends on the pressure limitation and on the chest compliance [20]. The decelerating waveform pattern leads to higher Pmean [21]. The shape of waveform is important. Both PCV and VCV with a decelerating flow waveform provide a lower PIP and higher Pmean compared to VCV with a square flow waveform [20, 22]. In addition it was shown in rabbits that high inspiratory flow rates with PCV yielded hypoxia by causing severe lung damage compared to low flow rate VCV [23]. This in turn, emphasizes the importance of flow rate.
In studies comparing PCV with VCV, airway pressures showed differences based on patient groups and type of surgery. Ppeak and Pplateau were lower (similar to Pmean) in patients receiving PCV compared to VCV in a study of adults undergoing one-lung ventilation [24]. Pplateau and Pmean were similar with PCV and VCV in obese patients in bariatric surgery [19]. In another study of laparoscopic surgery Ppeak was lower, but Pmean was higher with PCV compared to VCV [25]. In children during laparoscopic surgery, Pmean was higher although Ppeak was similar with PCV compared to VCV [26].
Although PCV is a preferred ventilation mode in order to prevent lung damage associated with airway pressure during ventilation, Prella M et al. [27] did not find any difference in incidence of barotrauma in spite of higher Ppeak with VCV. While there is a weak relation between Ppeak and incidence of barotrauma, there is a strong relation between Ppeak and ventilator-induced barotrauma when Pplateau exceed 35cmH20 [28]. Therefore, maintaining the Pplateau below 30 cmH20 during VCV can minimize lung damage [29].
Favorable effects of PCV on improving oxygenation in patients with ARDS have already been mentioned. In these studies, different I/E ratios along with prolonged inspiration times and inverse ratio ventilation (IRV) were used. It was suggested that the effect of PCV on improving oxygenation could arise from alveolar recruitment by forming intrinsic PEEP [30–32]. We did not measure intrinsic PEEP level in our study. This could be one of limitations of our study. In some studies, however, better oxygenation with PCV was linked with improvement of the ventilation/perfusion ratio due to homogenous gas distribution to various regions of the lung [22, 27, 33]. There are also studies suggesting that improved oxygenation levels are associated with increased Pmean [34]. We have managed to achieve normal ETCO2 levels with similar RR in both groups. Since we set FiO2 at 0.4 for all the patients, and as FiO2 and Ti/Ttot were similar in both groups, we believe that the reason for better oxygenation with PCV can be increased Pmean.
Mechanical ventilation in open cardiac surgery may be challenging for anesthetists. CPB initiates a systemic inflammatory response syndrome characterized by the activation of complement, neutrophils, endotoxin and the proinflammatory cytokines [35, 36]. Zupancich et al. [37] demonstrated in their study that ventilating with high TV/low PEEP was associated with inflammatory responses in comparison to low TV/high PEEP in patients receiving cardiac surgery that may attenuate lung injury. Koner et al. [38] administered 5cmH2O PEEP in addition to 6 mL/kg TV and 10cmH20 PEEP in addition to 6 mL/kg TV, and found similar levels of TNF alpha and IL-6. Comparing high TV and ZEEP mechanical ventilation with low TV and PEEP ventilation in patients during major surgery, the authors found no differences in pulmonary or systemic levels of inflammatory markers depending on ventilatory strategy [39]. In our study, we did not apply any extrinsic PEEP, to avoid the introduction of an additional confounding parameter. We performed total vital capacity maneuver (TVCM), which is our routine clinical practice, to all patients before termination of CBP. TVCM was performed by inflating the lungs to 40 cm H2O and holding this pressure for 15 s immediately before termination of CPB [40]. It was shown that postoperative atelectasis formation and intrapulmonary shunting could be completely prevented in the experimental studies by performing TVCM [41].
In mechanical ventilation, airway pressures, inspiratory flow rate, autoPEEP can lead to hypotension by lowering CO [42]. On the other hand, regulation of regional circulation with local factors such as improvement in CO and hypoxic pulmonary vasoconstriction can decrease intrapulmonary shunts and thus can improve oxygenation. The cardiovascular effects of mechanic ventilation are closely related to Pmean, through its effects on pleural pressure. Increasing of the Ti fraction with IRV may result in a significant increase in Pmean and may further impede venous return [34]. The impact of mechanical ventilation on SV depends on intravascular volume [43]. On the other hand, there are studies in which similar hemodynamic responses were obtained despite different static and dynamic airway pressures in PCV and VCV [14, 19, 44, 45]. In our study, we also attained similar hemodynamic response with PCV at the end of the surgery despite high Pmean; and this can be attributed to the fact that patients had normal cardiac functions and to sufficient intravascular volume.
We conducted this study only on patients with intact lung function. A similar study might be required to be done on patients with low respiratory reserve. In these patients, airway inflammation associated with CPB can be further worsened in presence of chronic obstructive pulmonary disease (COPD) [46]. Although PCV seems to be more convenient in patients with COPD because of high airway pressures associated with bronchoconstriction and secretion, no advantages of PCV or VCV over each other could be demonstrated in these patients [47].
Respiratory strategy in open heart surgery requires a multidisciplinary approach beginning with induction of anesthesia and continuing in the ICU. Regardless of the mode of ventilation that is chosen, attention to the determinants of Pmean and oxygenation is important [48].
As a result, better oxygenation was achieved, PaO2/FIO2 ratio was increased, and time to extubation and ICU length of stay were shortened in patients who underwent open heart surgery with PCV compared to VCV. Both ventilation modes affected the hemodynamics similarly. PVC may be preferable to VCV in patients who undergo open heart surgery. However, it would be convenient if our findings are supported by similar studies.
References
Weissman C. Pulmonary complications after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8(3):185–211.
Welsby IJ, Bennett-Guerrero E, Atwell D, White WD, Newman MF, Smith PK, et al. The association of complication type with mortality and prolonged stay after cardiac surgery with cardiopulmonary bypass. Anesth Analg. 2002;94(5):1072–8.
Weiss YG, Merin G, Koganov E, Ribo A, Oppenheim-Eden A, Medalion B, et al. Postcardiopulmonary bypass hypoxemia: a prospective study on incidence, risk factors, and clinical significance. J Cardiothorac Vasc Anesth. 2000;14(5):506–13.
Dyhr T, Laursen N, Larsson A. Effects of lung recruitment maneuver and positive end-expiratory pressure on lung volume, respiratory mechanics and alveolar gas mixing in patients ventilated after cardiac surgery. Acta Anaesthesiol Scand. 2002;46(6):717–25.
Barbosa RAG, Carmona MJC. Avaliação da função pulmonar em pacientes submetidos à cirurgia cardíaca com circulação extracorpórea. Rev Bras Anestesiol. 2002;52(6):689–99.
Rodrigues CDA, Oliveira RARA, Soares SMTP, Figueiredo LC, Araújo S, Dragosavac D. Lung injury and mechanical ventilation in cardiac surgery: a review. Rev Bras Ter Intensiva. 2010;22(4):375–83.
Esteban A, Alía I, Gordo F, de Pablo R, Suarez J, González G, Blanco J. Prospective randomized trial comparing pressure-controlled ventilation and volume-controlled ventilation in ARDS. For the Spanish Lung Failure Collaborative Group. Chest. 2000;117(6):1690–6.
Ferrando C, Soro M, Belda FJ. Protection strategies during cardiopulmonary bypass: ventilation, anesthetics and oxygen. Curr Opin Anaesthesiol. 2015;28(1):73–80.
Nichols D, Haranath S. Pressure control ventilation. Crit Care Clin. 2007;23(2):183–99.
Samantaray A, Hemanth N. Comparison of two ventilation modes in post-cardiac surgical patients. Saudi J Anaesth. 2011;5(2):173–8.
Milot J, Perron J, Lacasse Y, Letourneau L, Cartier PC, Maltais F. Incidence and predictors of ARDS after cardiac surgery. Chest. 2001;119(3):884–8.
Christenson JT, Aeberhard JM, Badel P, Pepcak F, Maurice J, Simonet F, et al. Adult respiratory distress syndrome after cardiac surgery. Cardiovasc Surg. 1996;4:15–21.
Lopez-Delgado JC, Esteve F, Javierre C, Torrado H, Rodriguez-Castro D, Carrio ML, et al. Evaluation of the PaO2/FiO2 ratio after cardiac surgery as a predictor of outcome during hospital stay. BMC Anesthesiol. 2014;14:83.
Lessard MR, Guérot E, Lorino H, Lemaire F, Brochard L. Effects of pressure-controlled with different I: E ratios versus volume-controlled ventilation on respiratory mechanics, gas exchange, and hemodynamics in patients with adult respiratory distress syndrome. Anesthesiology. 1994;80(5):983–91.
Abraham E, Yoshihara G. Cardiorespiratory effects of pressure controlled ventilation in severe respiratory failure. Chest. 1990;98(6):1445–9.
Rappaport SH, Shpiner R, Yoshihara G, Wright J, Chang P, Abraham E. Randomized, prospective trial of pressure-limited versus volume-controlled ventilation in severe respiratory failure. Crit Care Med. 1994;22(1):22–32.
Doig GS, Simpson F. Randomization and allocation concealment: a practical guide for researchers. J Crit Care. 2005;20(2):187–91 (discussion 191–3).
The ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8.
Cadi P, Guenoun T, Journois D, Chevallier JM, Diehl JL, Safran D. Pressure-controlled ventilation improves oxygenation during laparoscopic obesity surgery compared with volume-controlled ventilation. Br J Anaesth. 2008;100(5):709–16.
Davis K Jr, Branson RD, Campbell RS, Porembka DT. Comparison of volume control and pressure control ventilation: Is flow waveform the difference? J Trauma. 1996;41(5):808–14.
McKibben AW, Ravenscraft SA. Pressure-controlled and volume-cycled mechanical ventilation. Clin Chest Med. 1996;17(3):395–410.
Al-Saady N, Bennett ED. Decelerating inspiratory flow waveform improves lung mechanics and gas exchange in patients on intermittent positive-pressure ventilation. Intensive Care Med. 1985;11(2):68–75.
Maeda Y, Fujino Y, Uchiyama A, Matsuura N, Mashimo T, Nishimura M. Effects of peak inspiratory flow on development of ventilator-induced lung injury in rabbits. Anesthesiology. 2004;101(3):722–8.
Tuğrul M, Camci E, Karadeniz H, Sentürk M, Pembeci K, Akpir K. Comparison of volume controlled with pressure controlled ventilation during one-lung anaesthesia. Br J Anaesth. 1997;79(3):306–10.
Balick-Weber CC, Nicolas P, Hedreville-Montout M, Blanchet P, Stéphan F. Respiratory and haemodynamic effects of volume-controlled vs pressure-controlled ventilation during laparoscopy: a cross-over study with echocardiographic assessment. Br J Anaesth. 2007;99(3):429–35.
Kim JY, Shin CS, Lee KC, Chang YJ, Kwak HJ. Effect of pressure-versus volume-controlled ventilation on the ventilatory and hemodynamic parameters during laparoscopic appendectomy in children: a prospective, randomized study. J Laparoendosc Adv Surg Tech A. 2011;21(7):655–8.
Prella M, Feihl F, Domenighetti G. Effects of short-term pressure-controlled ventilation on gas exchange, airway pressures, and gas distribution in patients with acute lung injury/ARDS: comparison with volume-controlled ventilation. Chest. 2002;122(4):1382–8. Erratum in: Chest. 2003;123(1):315.
Boussarsar M, Thierry G, Jaber S, Roudot-Thoraval F, Lemaire F, Brochard L. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med. 2002;28(4):406–13.
MacIntyre NR. Setting the frequence-tidal volume pattern. Respir Care. 2002;47(3):266–74 (discussion 274–8).
Cole AG, Weller SF, Sykes MK. Inverse ratio ventilation compared with PEEP in adult respiratory failure. Intensive Care Med. 1984;10(5):22–32.
Tharratt RS, Allen RP, Albertson TE. Pressure controlled inverse ratio ventilation in severe adult respiratory failure. Chest. 1988;94(4):755–62.
Gurevitch MJ, Van Dyke J, Young ES, Jackson K. Improved oxygenation and lower peak airway pressure in severe adult respiratory distress syndrome. Treatment with inverse ratio ventilation. Chest. 1986;89(2):211–3.
Garnero AJ, Abbona H, Gordo-Vidal F, Hermosa-Gelbard C, Grupo de Insuficiencia Respiratoria Aguda de SEMICYUC. Pressure versus volume controlled modes in invasive mechanical ventilation. Med Intensiva. 2013;37(4):292–8.
Marinii JJ, Ravenscraft SA. Mean airway pressure: physiologic determinants and clinical importance. Part II. Clinical implications. Crit Care Med. 1992;20(11):1604–16.
Butler J, Rocker GM, Westaby S. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1993;55(2):552–9.
Wan S, LeClerc JL, Vincent JL. Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest. 1997;112(3):676–92.
Zupancich E, Paparella D, Turani F, Munch C, Rossi A, Massaccesi S, Ranieri VM. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. J Thorac Cardiovasc Surg. 2005;130(2):378–83.
Koner O, Celebi S, Balci H, Cetin G, Karaoglu K, Cakar N. Effects of protective and conventional mechanical ventilation on pulmonary function and systemic cytokine release after cardiopulmonary bypass. Intensive Care Med. 2004;30(4):620–6.
Wrigge H, Uhlig U, Zinserling J, Behrends-Callsen E, Ottersbach G, Fischer M, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg. 2004;98(3):775–81.
Tschernko EM, Bambazek A, Wisser W, Partik B, Jantsch U, Kubin K, et al. Intrapulmonary shunt after cardiopulmonary bypass: the use of vital capacity maneuvers versus off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2002;124(4):732–8.
Magnusson L, Zemgulis V, Tenling A, Wernlund J, Tydén H, Thelin S. Use of a vital capacity maneuver to prevent atelectasis after cardiopulmonary bypass: an experimental study. Anesthesiology. 1998;88(1):134–42.
Qvist J, Pontoppidan H, Wilson RS, Lowenstein E, Laver MB. Hemodynamic responses to mechanical ventilation with PEEP: the effect of hypervolemia. Anesthesiology. 1975;42:45.
Pinsky MR. Heart-lung interactions. Curr Opin Crit Care. 2007;13(5):528–31.
Wang JP, Wang HB, Liu YJ, Lou XP, Wang XD, Kong Y. Comparison of pressure-and volume-controlled ventilation in laparoscopic surgery: a meta-analysis of randomized controlled trial. Clin Invest Med. 2015;38(3):E119–41.
Unzueta MC, Casas JI, Moral MV. Pressure-controlled versus volume-controlled ventilation during one-lung ventilation for thoracic surgery. Anesth Analg. 2007;104(5):1029–33.
Saleh HZ, Mohan K, Shaw M, Al-Rawi O, Elsayed H, Walshaw M, Chalmers JA, Fabri BM. Impact of chronic obstructive pulmonary disease severity on surgical outcomes in patients undergoing non-emergent coronary artery bypass grafting. Eur J Cardiothorac Surg. 2012;42(1):108–13.
Karakurt Z, Yarkin T, Altinöz H, Atik Güngör S, Adigüzel N, Güngör G, Demiryontar D, Biçakçi B, Berk Takir H, Unver E, Baran R. Pressure vs. volume control in COPD patients intubated due to ARF: a case-control study. Tuberk Toraks. 2009;57(2):145–54.
Tobias JD. Is there an optimal mode of ventilation following cardiac surgery? Saudi J Anaesth. 2011;5(2):121–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Hoşten, T., Kuş, A., Gümüş, E. et al. Comparison of intraoperative volume and pressure-controlled ventilation modes in patients who undergo open heart surgery. J Clin Monit Comput 31, 75–84 (2017). https://doi.org/10.1007/s10877-016-9824-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9824-x