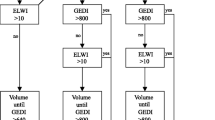
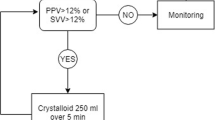
Abstract
Haemodynamic goal-directed therapies (GDT) may improve outcome following elective major surgery. So far, few data exist regarding haemodynamic optimization during emergency surgery. In this randomized, controlled trial, 50 surgical patients with hypovolemic or septic conditions were enrolled and we compared two algorithms of GDTs based either on conventional parameters and pressure pulse variation (control group) or on cardiac index, global end-diastolic volume index and stroke volume variation as derived from the PiCCO monitoring system (optimized group). Postoperative outcome was estimated by a composite index including major complications and by the Sequential Organ Failure Assessment (SOFA) Score within the first 3 days after surgery (POD1, POD2 and POD3). Data from 43 patients were analyzed (control group, N = 23; optimized group, N = 20). Similar amounts of fluid were given in the two groups. Intraoperatively, dobutamine was given in 45 % optimized patients but in no control patients. Major complications occurred more frequently in the optimized group [19 (95 %) versus 10 (40 %) in the control group, P < 0.001]. Likewise, SOFA scores were higher in the optimized group on POD1 (10.2 ± 2.5 versus 6.6 ± 2.2 in the control group, P = 0.001), POD2 (8.4 ± 2.6 vs 5.0 ± 2.4 in the control group, P = 0.002) and POD 3 (5.2 ± 3.6 and 2.2 ± 1.3 in the control group, P = 0.01). There was no significant difference in hospital mortality (13 % in the control group and 25 % in the optimized group). Haemodynamic optimization based on volumetric and flow PiCCO-derived parameters was associated with a less favorable postoperative outcome compared with a conventional GDT protocol during emergency surgery.


Similar content being viewed by others
References
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51.
Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–45.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensiv Care Med. 2013;39:165–228.
Wenzel V, Russo S, Arntz HR, Bahr J, Baubin MA, Bottiger BW, Dirks B, Dorges V, Eich C, Fischer M et al. Anaesthesist. 2006, 55:958–966, 968–972.
Rady MY, Rivers EP, Nowak RM. Resuscitation of the critically ill in the ED: responses of blood pressure, heart rate, shock index, central venous oxygen saturation, and lactate. Am J Emerg Med. 1996;14:218–25.
Ekbal NJ, Dyson A, Black C, Singer M. Monitoring tissue perfusion, oxygenation, and metabolism in critically ill patients. Chest. 2013;143:1799–808.
Fouche Y, Sikorski R, Dutton RP. Changing paradigms in surgical resuscitation. Crit Care Med. 2010;38(Suppl):S411–20.
Shoemaker WC, Montgomery ES, Kaplan E, Elwyn DH. Physiologic patterns in surviving and nonsurviving shock patients. Use of sequential cardiorespiratory variables in defining criteria for therapeutic goals and early warning of death. Arch Surg. 1973;106:630–6.
Shoemaker WC, Mohr PA, Printen KJ, Brown RS, Amato JJ, Carey JS, Youssef S, Reinhard JM, Kim SI, Kark AE. Use of sequential physiologic measurements for evaluation and therapy of uncomplicated septic shock. Surg Gynecol Obst. 1970;131:245–54.
Tuchschmidt J, Fried J, Astiz M, Rackow E. Elevation of cardiac output and oxygen delivery improves outcome in septic shock. Chest. 1992;102:216–20.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;34:1368–77.
Preisman S, Pfeiffer U, Lieberman N, Perel A. New monitors of intravascular volume: a comparison of arterial pressure waveform analysis and the intrathoracic blood volume. Intensiv Care Med. 1997;23:651–7.
Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–8.
Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO Jr, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11:R100.
Lobo SM, de Oliveira NE. Clinical review: what are the best hemodynamic targets for noncardiac surgical patients? Crit Care. 2013;17:210.
Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, Manthous C, Meduri GU, Moreno RP, Putensen C, Stewart T, et al. Hemodynamic monitoring in shock and implications for management. International consensus conference, Paris, France, 27–28 April 2006. Intensiv Care Med. 2007;33:575–90.
Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Crit Care. 2005;9:R687–93.
Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, Grocott MP, Ahern A, Griggs K, Scott R, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–90.
Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002;30:1686–92.
Arulkumaran N, Corredor C, Hamilton MA, Ball J, Grounds RM, Rhodes A, Cecconi M. Cardiac complications associated with goal-directed therapy in high-risk surgical patients: a meta-analysis. Br J Anaesth. 2014;112:648–59.
Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, Hamilton M, Rhodes A. Clinical review: goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17:209.
Dunser MW, Ruokonen E, Pettila V, Ulmer H, Torgersen C, Schmittinger CA, Jakob S, Takala J. Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Crit Care. 2009;13:R181.
Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–22.
Wilms H, Mittal A, Haydock MD, van den Heever M, Devaud M, Windsor JA. A systematic review of goal directed fluid therapy: rating of evidence for goals and monitoring methods. J Crit Care. 2014;29:204–9.
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
Kobayashi L, Costantini TW, Coimbra R. Hypovolemic shock resuscitation. Surg Clin N Am. 2012;92:1403–23.
Williams DJ, Walker JD. A nomogram to calculate the physiological and operative severity score for the enUmeration of Mortality and morbidity (POSSUM). Br J Surg. 2014;101:239–45.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Fueglistaler P, Amsler F, Schuepp M, Fueglistaler-Montali I, Attenberger C, Pargger H, Jacob AL, Gross T. Prognostic value of sequential organ failure assessment and simplified acute physiology II score compared with trauma scores in the outcome of multiple-trauma patients. Am J Surg. 2010;200:204–14.
Ochiai T, Hiranuma S, Takiguchi N, Ito K, Kawaguchi A, Iwai T, Arii S. SOFA score predicts postoperative outcome of patients with colorectal perforation. Hepatogastroenterology. 2004;51:1007–10.
Lees N, Hamilton M, Rhodes A. Clinical review: goal-directed therapy in high risk surgical patients. Crit Care. 2009;13(5):231.
Boland MR, Noorani A, Varty K, Coffey JC, Agha R, Walsh SR. Perioperative fluid restriction in major abdominal surgery: systematic review and meta-analysis of randomized, clinical trials. World J Surg. 2013;37:1193–202.
Grocott MP, Dushianthan A, Hamilton MA, Mythen MG, Harrison D, Rowan K. Optimisation systematic review steering G: perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: a cochrane systematic review. Br J Anaesth. 2013;111:535–48.
Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB, Minto G. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth. 2012;108:53–62.
Ghneim MH, Regner JL, Jupiter DC, Kang F, Bonner GL, Bready MS, Frazee R, Ciceri D, Davis ML. Goal directed fluid resuscitation decreases time for lactate clearance and facilitates early fascial closure in damage control surgery. Am J Surg. 2013;206:995–9.
Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009;37(10):2827–39.
Vincent JL, Ferreira F, Moreno R. Scoring systems for assessing organ dysfunction and survival. Crit Care Clin. 2000;16:353–66.
Meyer ZC, Schreinemakers JM, Mulder PG, de Waal RA, Ermens AA, van der Laan L. The role of C-reactive protein and the SOFA score as parameter for clinical decision making in surgical patients during the intensive care unit course. PLoS One. 2013;8:e55964.
Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–402.
Harten J, Crozier JE, McCreath B, Hay A, McMillan DC, McArdle CS, Kinsella J. Effect of intraoperative fluid optimisation on renal function in patients undergoing emergency abdominal surgery: a randomised controlled pilot study (ISRCTN 11799696). Int J Surg. 2008;6:197–204.
Licker M, Diaper J, Villiger Y, Spiliopoulos A, Licker V, Robert J, Tschopp JM. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care. 2009;13:R41.
Takala J, Ruokonen E, Tenhunen JJ, Parviainen I, Jakob SM. Early non-invasive cardiac output monitoring in hemodynamically unstable intensive care patients: a multi-center randomized controlled trial. Crit Care. 2011;15:R148.
Takala J, Meier-Hellmann A, Eddleston J, Hulstaert P, Sramek V. Effect of dopexamine on outcome after major abdominal surgery: a prospective, randomized, controlled multicenter study. European multicenter study group on dopexamine in major abdominal surgery. Crit Care Med. 2000;28:3417–23.
Hernandez G, Bruhn A, Luengo C, Regueira T, Kattan E, Fuentealba A, Florez J, Castro R, Aquevedo A, Pairumani R, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensiv Care Med. 2013;39:1435–43.
De Backer D, Creteur J, Dubois MJ, Sakr Y, Koch M, Verdant C, Vincent JL. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006;34:403–8.
Shahin J, DeVarennes B, Tse CW, Amarica DA, Dial S. The relationship between inotrope exposure, six-hour postoperative physiological variables, hospital mortality and renal dysfunction in patients undergoing cardiac surgery. Crit Care. 2011;15:R162.
Nielsen DV, Hansen MK, Johnsen SP, Hansen M, Hindsholm K, Jakobsen CJ. Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology. 2014;120:1098–108.
Tacon CL, McCaffrey J, Delaney A. Dobutamine for patients with severe heart failure: a systematic review and meta-analysis of randomised controlled trials. Intensiv Care Med. 2012;38:359–67.
Rhodes SS, Ropella KM, Camara AK, Chen Q, Riess ML, Pagel PS, Stowe DF. Ischemia-reperfusion injury changes the dynamics of Ca2+—contraction coupling due to inotropic drugs in isolated hearts. J Appl Physiol. 2006;100:940–50.
Zhou L, Huang H, Yuan CL, Keung W, Lopaschuk GD, Stanley WC. Metabolic response to an acute jump in cardiac workload: effects on malonyl-CoA, mechanical efficiency, and fatty acid oxidation. Am J Physiol Heart Circ Physiol. 2008;294:H954–60.
Singer M. Catecholamine treatment for shock—equally good or bad? Lancet. 2007;370:636–7.
Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, Orecchioni A, D’Egidio A, D’Ippoliti F, Raffone C, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310:1683–91.
Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108:887–97.
Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: ClinicalTrial.gov NCT0165-3977.
Appendix
Appendix
Severe Hypovolemia corresponding to class III or IV hemorrhagic shock according to the following criteria:
-
HR > 100 mmHg with systolic arterial pressure (SAP) < 100 mmHg after fluid loading (10 ml/kg) or with a blood lactate level > 4mm/L
Severe sepsis refers to sepsis-induced tissue hypoperfusion or organ dysfunction with any of the following thought to be due to the infection:
-
Sepsis-induced hypotension (SAP < 90 mmHg or MAP < 70 mmHg or SAP decrease more than 40 mmHg or two standard deviations below normal age in the absence of other causes of hypotension
-
Lactate above upper limits of laboratory normal
-
Urine output <0.5 mL/kg/hr for more than 2 h despite adequate fluid resuscitation
-
Acute lung injury with PaO2/FIO2 < 250 in the absence of pneumonia as infection source
-
Acute lung injury with PaO2/FIO2 < 200 in the presence of pneumonia as infection source
-
Creatinine > 2 mg/dL (176.8 μmol/L)
-
Bilirubin >4 mg/dL (34.2 μmol/L)
-
Platelet count <100,000 μL–1
-
Coagulopathy (INR > 1.5)
Sepsis-induced hypotension is defined as a systolic blood pressure (SBP) <90 mmHg or MAP <70 mmHg or a SBP decrease >40 mmHg or less than two standard deviations below normal for age in the absence of other causes of hypotension.
Rights and permissions
About this article
Cite this article
Pavlovic, G., Diaper, J., Ellenberger, C. et al. Impact of early haemodynamic goal-directed therapy in patients undergoing emergency surgery: an open prospective, randomised trial. J Clin Monit Comput 30, 87–99 (2016). https://doi.org/10.1007/s10877-015-9691-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-015-9691-x