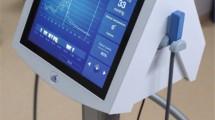
Abstract
The recently developed technique for measuring cutaneous mitochondrial oxygen tension (mitoPO2) by means of the Protoporphyrin IX—Triplet State Lifetime Technique (PpIX-TSLT) provides new opportunities for assessing mitochondrial function in vivo. The aims of this work were to study whether cutaneous mitochondrial measurements reflect mitochondrial status in other parts of the body and to demonstrate the feasibility of the technique for potential clinical use. The first part of this paper demonstrates a correlation between alterations in mitochondrial parameters in skin and other tissues during endotoxemia. Experiments were performed in rats in which mitochondrial dysfunction was induced by a lipopolysaccharide-induced sepsis (n = 5) and a time control group (n = 5). MitoPO2 and mitochondrial oxygen consumption (mitoVO2) were measured using PpIX-TSLT in skin, liver and buccal mucosa of the mouth. Both skin and buccal mucosa show a significant mitoPO2-independent decrease (P < 0.05) in mitoVO2 after LPS infusion (a decrease of 37 and 39 % respectively). In liver both mitoPO2 and mitoVO2 decreased significantly (33 and 27 % respectively). The second part of this paper describes the clinical concept of monitoring cutaneous mitochondrial respiration in man. A first prototype of a clinical PpIX-TSLT monitor is described and its usability is demonstrated on human skin. We expect that clinical implementation of this device will greatly contribute to our understanding of mitochondrial oxygenation and oxygen metabolism in perioperative medicine and in critical illness. Our ultimate goal is to develop a clinical monitor for mitochondrial function and the current results are an important step forward.







Similar content being viewed by others
References
Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38(10):1278–95.
Schapira AH, Gegg M. Mitochondrial contribution to Parkinson’s disease pathogenesis. Parkinsons Dis. 2011;2011:159160.
Fink M. Cytopathic hypoxia in sepsis. Acta Anaesthesiol Scand Suppl. 1997;110:87–95.
Fink MP. Cytopathic hypoxia. A concept to explain organ dysfunction in sepsis. Minerva Anestesiol. 2000;66(5):337–42.
Fink MP. Cytopathic hypoxia in sepsis: a true problem? Minerva Anestesiol. 2001;67(4):290–1.
Wagner F, et al. Sepsis therapy: what’s the best for the mitochondria? Crit Care. 2008;12(4):171.
Galley HF. Bench-to-bedside review: targeting antioxidants to mitochondria in sepsis. Crit Care. 2010;14(4):230.
Fontaine EM, et al. Cytoplasmic cellular structures control permeability of outer mitochondrial membrane for ADP and oxidative phosphorylation in rat liver cells. Biochem Biophys Res Commun. 1995;213(1):138–46.
Saks V, et al. Correlation between degree of rupture of outer mitochondrial membrane and changes of kinetics of regulation of respiration by ADP in permeabilized heart and liver cells. Biochem Biophys Res Commun. 1995;208(3):919–26.
Brealey D, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R491-7.
Crouser ED, et al. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit Care Med. 2002;30(2):276–84.
Gellerich FN, et al. Impaired energy metabolism in hearts of septic baboons: diminished activities of complex I and complex II of the mitochondrial respiratory chain. Shock. 1999;11(5):336–41.
Mik EG, et al. In vivo mitochondrial oxygen tension measured by a delayed fluorescence lifetime technique. Biophys J. 2008;95(8):3977–90.
Mik EG, et al. Mitochondrial PO2 measured by delayed fluorescence of endogenous protoporphyrin IX. Nat Methods. 2006;3(11):939–45.
Mik EG, et al. Mitochondrial oxygen tension within the heart. J Mol Cell Cardiol. 2009;46(6):943–51.
Harms FA, et al. Oxygen-dependent delayed fluorescence measured in skin after topical application of 5-aminolevulinic acid. J Biophotonics. 2011;4(10):731–9.
Choi JK, et al. Evaluation of mean skin temperature formulas by infrared thermography. Int J Biometeorol. 1997;41(2):68–75.
Mik EG. Methods and devices for assessment of mitochondrial function. 2009: US 2011/0182825 A1.
Mik EG. Special article: measuring mitochondrial oxygen tension: from basic principles to application in humans. Anesth Analg. 2013;117(4):834–46.
Buerk DG, et al. Interpretation of oxygen disappearance curves measured in blood perfused tissues. Adv Exp Med Biol. 1986;200:151–61.
Gaab MR, Poch B, Heller V. Oxygen tension, oxygen metabolism, and microcirculation in vasogenic brain edema. Adv Neurol. 1990;52:247–56.
Golub AS, Tevald MA, Pittman RN. Phosphorescence quenching microrespirometry of skeletal muscle in situ. Am J Physiol Heart Circ Physiol. 2011;300(1):H135–43.
Reneau DD, Halsey JH Jr. Interpretation of oxygen disappearance rates in brain cortex following total ischaemia. Adv Exp Med Biol. 1977;94:189–98.
Harms FA, et al. Cutaneous respirometry by dynamic measurement of mitochondrial oxygen tension for monitoring mitochondrial function in vivo. Mitochondrion. 2013;13(5):507–14.
Golub AS, et al. Analysis of phosphorescence in heterogeneous systems using distributions of quencher concentration. Biophys J. 1997;73(1):452–65.
Bodmer SI, et al. Microvascular and mitochondrial PO(2) simultaneously measured by oxygen-dependent delayed luminescence. J Biophotonics. 2012;5(2):140–51.
Mik EG, Johannes T, Ince C. Monitoring of renal venous PO2 and kidney oxygen consumption in rats by a near-infrared phosphorescence lifetime technique. Am J Physiol Renal Physiol. 2008;294(3):F676–81.
Directive 2006/25/EC of the European parliament and the council of 5 April 2006 on the (artificial optical radiation). E.p.a.t.c.o.A. 2006, Editor. 2006.
EN 60601-1:2006; NB-MED. 2012-01-24.
Harms FA et al. Cutaneous respirometry by dynamic measurement of mitochondrial oxygen tension for monitoring mitochondrial function in vivo. Mitochondrion 2013;13(5):507–14.
Fredriksson K, Rooyackers O. Mitochondrial function in sepsis: respiratory versus leg muscle. Crit Care Med. 2007;35(9 Suppl):S449–53.
Poderoso JJ, et al. Liver oxygen uptake dependence and mitochondrial function in septic rats. Circ Shock. 1994;44(4):175–82.
Llesuy S, et al. Oxidative stress in muscle and liver of rats with septic syndrome. Free Radic Biol Med. 1994;16(4):445–51.
Geller ER, Jankauskas S, Kirkpatrick J. Mitochondrial death in sepsis: a failed concept. J Surg Res. 1986;40(5):514–7.
Garrison RN, Ratcliffe DJ, Fry DE. The effects of peritonitis on murine renal mitochondria. Adv Shock Res. 1982;7:71–6.
Singer M. Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med. 2007;35(9 Suppl):S441–8.
Rosser DM, et al. Endotoxin reduces maximal oxygen consumption in hepatocytes independent of any hypoxic insult. Intensive Care Med. 1998;24(7):725–9.
Harms FA, et al. Validation of the protoporphyrin IX-triplet state lifetime technique for mitochondrial oxygen measurements in the skin. Opt Lett. 2012;37(13):2625–7.
Mik EG. Measuring mitochondrial oxygen tension: from basic principles to application in humans. Anesth Analg. 2013.
Ibbotson SH. Adverse effects of topical photodynamic therapy. Photodermatol Photoimmunol Photomed. 2011;27(3):116–30.
Acknowledgments
This work was financially supported by a Life Sciences Pre-Seed Grant (Grant No. 40-41300-98-9037) from the Netherlands Organization for Health Research and Development (ZonMW).
Conflict of interest
Dr. E.G. Mik is founder and shareholder of Photonics Healthcare B.V., a company aimed at making the delayed fluorescence lifetime technology clinically available. Photonics Healthcare B.V. holds the exclusive licenses to several patents regarding this technology, filed and owned by the Academic Medical Center in Amsterdam and the Erasmus Medical Center in Rotterdam, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harms, F.A., Bodmer, S.I.A., Raat, N.J.H. et al. Cutaneous mitochondrial respirometry: non-invasive monitoring of mitochondrial function. J Clin Monit Comput 29, 509–519 (2015). https://doi.org/10.1007/s10877-014-9628-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-014-9628-9