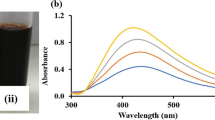
Abstract
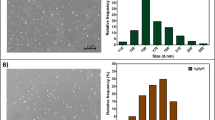
In this study, silver nanoparticle was green synthesized using the leaf extract of Solanum nigrum (Sn-AgNPs) and bio-physically characterized by UV–Vis spectroscopy, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), High resolution transmission electron microscopy (HR-TEM), Zeta potential analysis and Energy dispersive X-ray (EDX) analysis. The ecotoxicity of silver nanoparticle (Sn-AgNPs) were tested against both invertebrate (Ceriodaphnia cornuta and Paramecium sp.) and vertebrate aquatic animal models (Guppy fish, Poecilia reticulata) in comparison with bare silver nitrate. Sn-AgNPs were observed to be less toxic than ionic silver (silver nitrate). The ecotoxicity levels of Sn-AgNPs were found to be varied between tested organisms. Sn-AgNPs caused 100% mortality of freshwater crustacean, C. cornuta at 50 µg mL−1. At concentration below 50 µg mL−1 (10–30 µg mL−1), abnormality in the swimming behavior of C. cornuta was noticed. The ingestion and accumulation of Sn-AgNPs in the intestine of C. cornuta neonates were visualized under light and confocal laser scanning microscopic images. The ecotoxicity of Sn-AgNPs to the freshwater protozoan ciliate, Paramecium sp. showed that 30 µg mL−1 were lethal and produced 100% mortality at the same concentration. The study concludes that Sn-AgNPs was less toxic to both invertebrate and vertebrate models compared to ionic silver nitrate.









Similar content being viewed by others
References
J. Zhao and V. Castranova (2011). J. Toxicol. Environ. Health B14, 593–632.
K. Aschberger, C. Micheletti, B. Sokull-Kluttgen, and F. M. Christensen (2011). Environ. Int.37, 1143–1156.
C. Blaise, F. Gagne, and J. F. Ferard (2008). Environ. Toxicol.23, 591–598.
M. Farre, K. Gajda-Schrantz, L. Kantiani, and D. Barcelo (2009). Anal. Bioanal. Chem.393, 81–95.
A. Bermejo-Nogales, M. Fernández, M. L. Fernández-Cruz, and J. M. Navas (2016). Comp. Biochem. Physiol. C.190, 54–65.
F. Gottschalk, T. Y. Sun, and B. Nowack (2013). Environ. Poll.181, 287–300.
K. L. Garner, S. Suh, H. S. Lenihan, and A. A. Keller (2015). Environ. Sci. Technol.49, 5753–5759.
A. H. Sayed and H. A. M. Soliman (2017). Mutat. Res. Gen. Tox. En.822, 34–40.
K. Chaloupka, Y. Malam, and A. M. Seifalian (2010). Trends. Biotechnol.28, 580–588.
T. M. Benn and P. Westerhoff (2008). Environ. Sci. Technol.42, 4133–4139.
Q. Chaudhry, M. Scotter, J. Blackburn, B. Ross, A. Boxall, L. Castle, R. Aitken, and R. Watkins (2008). Food. Addit. Contam.25, 241–258.
L. Geranio, M. Heuberger, and B. Nowack (2009). Environ. Sci. Technol.43, 8113–8118.
R. Kaegi, B. Sinnet, S. Zuleeg, H. Hagendorfer, E. Mueller, R. Vonbank, M. Boller, and M. Burkhardt (2010). Environ. Pollut.158, 2900–2905.
A. A. Keller, S. McFerran, A. Lazareva, and S. Suh (2013). J. Nanopart. Res.15, 1–17.
T.F. Rozan, K.S. Hunter and G. Benoit (1995). Proceedings of the 3rd Argentum International Conference on the Transport, Fate and Effects of Silver in the Environment, Washington, DC, USA, August 6–9, pp. 181–184.
L. S. Wen, P. H. Santschi, G. A. Gill, C. L. Paternostro, and R. D. Lehman (1997). Environ. Sci. Technol.31, 723–731.
A. M. E. Badawy, T. P. Luxton, R. G. Silva, K. G. Scheckel, M. T. Suidan, and T. M. Tolaymat (2010). Environ. Sci. Technol.44, 1260–1266.
M. Tejamaya, I. Römer, R. C. Merrifield, and J. R. Lead (2012). Environ Sci Technol.46, 7011–7017.
I. Römer, T. A. White, M. Baalousha, K. Chipman, M. R. Viant, and J. R. Lead (2011). J. Chromatogr. A1218, 4226–4233.
S. A. Cumberland and J. R. Lead (2009). J. Chromatogr. A1216, 9099–9105.
M. Baalousha, Y. Nur, I. Römer, M. Tejamaya, and J. R. Lead (2013). Sci. Total Environ.454, 119–131.
M. Ahamed, M. Karns, M. Goodson, J. Rowe, S. M. Hussain, J. J. Schlager, and Y. Hong (2008). Toxicol Appl. Pharmacol.233, 404–410.
S. Arora, J. Jain, J. Rajwade, and K. Paknikar (2009). Toxicol. Appl. Pharmacol.236, 310–318.
P. AshaRani, M. P. Hande, and S. Valiyaveettil (2009). BMC Cell Biol.10, 65.
B. K. Gaiser, A. Biswas, P. Rosenkranz, M. A. Jepson, J. R. Lead, V. Stone, C. R. Tyler, and T. F. Fernandes (2011). J. Environ. Monit.13, 1227–1235.
J. Y. Roh, S. J. Sim, K. Yi, K. Park, K. H. Chung, D. Y. Ryu, and J. Choi (2009). Environ. Sci. Technol.43, 3933–3940.
OECD, (1984). 2–5.
EPA (2002).Ed Agency U S E P.
J. Bernal and S. Ruvalcaba (1996). Toxicology108, 165–173.
M. Ates, V. Demir, R. Adiguzel and Z. Arslan (2013).J. Nanomater. 1–6.
Z. A. Zakaria, H. K. Gopalan, and H. Zainal (2006). Yakugaku Zasshi.126, 1171–1178.
B. Malaikozhundan, B. Vaseeharan, S. Vijayakumar, R. Sudhakaran, N. Gobi, and G. Shanthini (2016). Biocatal. Agric. Biotechnol.8, 189–196.
B. Malaikozhundan, B. Vaseeharan, S. Vijayakumar, K. Pandiselvi, M. Rajamohamed Kalanjiam, K. Murugan, and G. Benelli (2017). Microb. Pathog.104, 268–277.
USEPA 2002.5th Edn. EPA-821-R-02-012.
S. Vijayakumar, B. Malaikozhundan, N. Gobi, B. Vaseeharan, and C. Murthy (2016). Limnologica.61, 44–51.
H. W. Bischoff and H. C. Bold (1983). Univ. Texas Publ.6318, 96.
H. Hosoya, K. Kimura, S. Matsuda, M. Kitamura, T. Takahashi, and T. Kosaka (1995). Zool. Sci.12, 807–810.
R. M. Shahjahan, M. J. Ahmed, R. A. Begunand, and M. A. Rashid (2013). J. Asiat. Soc. Bangladesh Sci.39, 259–267.
N. S. Taylor, R. J. Weber, A. D. Southam, T. G. Payne, O. Hrydziuszko, T. N. Arvanitis, and M. R. Viant (2009). Metabolomics.5, 44–58.
M. Yilmaz, A. Gül, and E. Karaköse (2004). Chemosphere.56, 375–380.
W. Fan, Q. Li, X. Yang, and L. Zhang (2013). PLOS ONE.8, 1–6.
M. R. Bindhu and M. Umadevi (2015). Spectrochim. Acta A Mol. Biomol. Spectrosc.135, 373–378.
S. Lokina, A. Stephen, V. Kaviyarasan, C. Arulvasu, and V. Narayanan (2014). Eur. J. Med. Chem.76, 256–263.
D. S. Kumar, K. V. Sharathnath, P. Yogeshwaran, A. Harani, K. Sudhakar, P. Sudha, and B. David (2010). Int. J. Pharm. Sci. Res.1, 95–100.
G. Leela Prakash, J. C. Rose, B. M. Gowtham, J. P. Krishna, and A. S. Prasad (2011). Pharmacophore2, 244–252.
C. A. Annapoorani (2013). Int. J. Pharm. Res. Dev.5, 01–06.
B. Ajitha, Y.A.K. Reddy and P.S. Reddy, J Photochem Photobiol B. 146, 1–9.
H. L. Su, C. C. Chou, D. J. Hung, S. H. Lin, I. C. Pao, J. H. Lin, F. L. Huang, R. X. Dong, and J. J. Lin (2009). Biomaterials.30, 5979–5987.
J. Fabrega, S. N. Luoma, C. R. Tyler, T. S. Galloway, and J. R. Lead (2011). Environ. Int.37, 517–531.
E. Oberdorster, S. Zhu, T. M. Blickley, P. McClellan-Green, and M. L. Haasch (2006). Carbon.44, 1112–1120.
K. M. Newton, H. L. Puppala, C. L. Kitchens, V. L. Colvin, and S. J. Klainey (2013). Environ. Toxicol. Chem.32, 2356–2364.
M. Heinlaan, A. Ivask, I. Blinova, H. C. Dubourguier, and A. Kahru (2008). Chemosphere.71, 1308–1316.
I. Blinova, A. Ivask, M. Heinlaan, M. Mortimer, and A. Kahru (2010). Environ. Pollut.158, 41–47.
R. J. Griffitt, J. Luo, J. Gao, J. C. Bonzongo, and D. S. Barber (2008). Environ. Toxicol. Chem.27, 1972–1978.
K. Bilberg, K. B. Doving, K. Beedholm, and E. Baatrup (2011). Aquat. Toxicol.104, 145–152.
L. K. Adams, D. Y. Lyon, A. McIntosh, and P. J. J. Alvarez (2006). Water Res.40, 3527–3532.
RH. Peters and R. De Bernardi (1987). Daphnia, Consiglionazionaledellerecherche´, Institutoitaliano di, idrobiologia, VerbaniaPallanza.
M. C. Artal, R. D. Holtz, F. Kummrow, O. L. Alves, and G. A. Umbuzeiro (2013). Environ. Toxicol. Chem.32, 908–912.
K. P. Tavares, A. Caloto-Oliveira, D. S. Vicentini, S. P. Melegari, W. G. Matias, S. Barbosa, and F. Kummrow (2014). Ecotoxicol. Environ. Contam.9, 43–50.
N. Strigul, L. Vaccari, C. Galdun, M. Wazne, X. Liu, C. Christodoulatos, and K. Jasinkiewicz (2009). Desalination248, 771–782.
M. Morange (2006). J Biosci.31, 27–30.
L. Kvitek, M. Vanickova, A. Panacek, J. Soukupova, M. Dittrich, E. Valentova, R. Prucek, M. Bancirova, D. Milde, and R. Zboril (2009). J. Phys. Chem. C113, 4296–4300.
J. García Alonso, F. R. Khan, S. K. Misra, M. Turmaine, B. D. Smith, P. S. Rainbow, S. N. Luoma, and E. Valsami-Jones (2011). Environ. Sci. Technol.45, 4630–4636.
A. V. Nebeker, C. K. McAuliffe, R. Mshar, and D. G. Stevens (1983). Environ. Toxicol. Chem.2, (9), 5–104.
A. Bianchini, M. Grosell, S. M. Gregory, and C. M. Wood (2002). Environ. Sci. Technol.36, 1763–1766.
T. P. Morgan and C. M. Wood (2004). Environ. Toxicol. Chem.23, 1261–1267.
J. A. Kovriznych, R. Sotníkova, D. Zeljenkova, E. Rollerova, E. Szabova, and S. Wimmerova (2013). Interdiscip. Toxicol.6, 67–73.
B. C. Lee, K. T. Kim, J. G. Cho, J. W. Lee, T. K. Ryu, J. H. Yoon, S. H. Lee, C. N. Duong, I. C. Eon, P. J. Kim, and K. H. Choi (2012). Mol. Cell. Toxicol.8, 357–366.
K. Bilberg, M.B. Hovgaard, F. Besenbacher and E. Baatrup (2012). J. Toxicol. 1–9.
P. V. Asharani, Y. I. Lian Wu, Z. Gong, and S. Valiyaveettil (2008). Nanotechnology19, 255102.
C. Fernandes, A. Fontaínhas-Fernandes, E. Rocha, and M. A. Salgado (2008). Environ. Monit. Assess.145, 315–322.
G. Federacy, B. J. Shaw, and R. D. Handy (2007). Aquat. Toxicol.84, 415–430.
Y. Wu and Q. Zhou (2013). Environ. Toxicol. Chem.32, 165–173.
K. J. Lee, P. D. Nallathamby, L. M. Browning, C. J. Osgood, and X. H. N. Xu (2007). ACS Nano1, 133–143.
Y. Min-Kyeong and K. Misook (2008). Bull. Korean Chem. Soc.29, 1179–1184.
R. J. Griffitt, K. N. D. Hyndman, N. D. Denslow, and D. S. Barber (2009). Toxicol. Sci.107, 404–415.
A. H. Ringwood, M. McCarthy, T. C. Bates, and D. L. Carroll (2010). Mar. Environ. Res.1, 49–51.
A.A. Hadi and S.F. Alwan, Int. J. Pharm. Life Sci.3, 2071–2081.
J. Pan and W. Wang (2004). Environ. Pollut.129, 467–477.
C. M. Zhao and W. X. Wang (2010). Environ. Sci. Technol.44, 7699–7704.
Acknowledgements
The authors thank the RUSA phase 2.0 grant [Ref-24-51-2014-U policy] TN Multi-Gen. Department of Education, Government of India. The corresponding author Dr. B. Vaseeharan thanks the Department of Biotechnology (DBT), New Delhi, India, for financial assistance under the Project Grants Code: BT/PR7903/AAQ/3/638/2013. The third author S. Vijayakumar (SRF) thanks the DST, New Delhi, India for financial support under INSPIRE programme (INSPIRE Fellow-IF140145). The authors gratefully acknowledge the University Scientific Instrumentation Centre (USIC) for providing Confocal laser scanning microscopy, XRD and FTIR instrumental facilities to this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jenifer, A.A., Malaikozhundan, B., Vijayakumar, S. et al. Green Synthesis and Characterization of Silver Nanoparticles (AgNPs) Using Leaf Extract of Solanum nigrum and Assessment of Toxicity in Vertebrate and Invertebrate Aquatic Animals. J Clust Sci 31, 989–1002 (2020). https://doi.org/10.1007/s10876-019-01704-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-019-01704-7