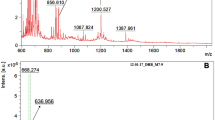
Abstract
Methyl vinyl ketone (MVK) is a key first-generation product from atmospheric isoprene photo-oxidation, especially under high-NOx conditions. In this work, acid-catalyzed reactions of gas-phase MVK with ammonium sulfate (AS), ammonium bisulfate (ABS), and sulfuric acid (SA) particles were investigated in a flow reaction system at relative humidity (RH) of 40 % and 80 %. Ultra-performance liquid chromatography with electrospray ionization time-of-flight mass spectrometry (UPLC/ESI-TOFMS) and gas chromatography-mass spectrometry (GC-MS) are utilized to identify particle-phase products for developing the reaction mechanisms. High-order oligomers such as dimers and tetramers were detected when ABS and SA particles were used, while no oligomeric products were found when AS particles were used. Particle-phase oligomeric products were formed via i) acid-catalyzed aldol reaction with or without dehydration and/or ii) acid-catalyzed hydration followed by oligomerization. Reactions on SA particles yield more abundant and higher-order oligomers up to hexamers than on ABS particles. Moreover, aldol reaction occurred only on SA particles, but hydration followed by oligomerization occurred in both ABS and SA particles. The high RH condition with the same type of acidic particles was found to favor hydration and facilitate the subsequent oligomerization, while the low RH condition with the same type of acidic particles was found to favor aldol reaction with dehydration (aldol condensation). Overall, the findings suggest acidic particles can facilitate the formation of high-order oligomers in the particle phase, with particle acidity and RH as key factors.







Similar content being viewed by others
References
Blank, I., Milo, C., Lin, J.M., Fay, L.B.: Quantification of aroma-impact components by isotope dilution assay — recent developments. Flavor chemistry: 30 years of progress. Kluwer Academic / Plenum Publishers, New York (1999)
Budzikiewicz, H., Drabner, G., Hammes, C.: Rearrangements accompanying the fragmentation of ionized 1-Phenylalkan-1-Ols. Org. Mass Spectrom. 28(11), 1326–1328 (1993)
Carlton, A.G., Wiedinmyer, C., Kroll, J.H.: A review of secondary organic aerosol (SOA) formation from isoprene. Atmos. Chem. Phys. 9(14), 4987–5005 (2009). doi:10.5194/acp-9-4987-2009
Chan, L.P., Chan, C.K.: Enhanced reactive uptake of nonanal by acidic aerosols in the presence of particle-phase organics. Aerosol Sci. Technol. 45(7), 872–883 (2011). doi:10.1080/02786826.2011.567314
Chan, A.W.H., Chan, M.N., Surratt, J.D., Chhabra, P.S., Loza, C.L., Crounse, J.D., Yee, L.D., Flagan, R.C., Wennberg, P.O., Seinfeld, J.H.: Role of aldehyde chemistry and NOx concentrations in secondary organic aerosol formation. Atmos. Chem. Phys. 10(15), 7169–7188 (2010). doi:10.5194/acp-10-7169-2010
Chen, Z.M., Jie, C.Y., Li, S., Wang, H.L., Wang, C.X., Xu, J.R., Hua, W.: Heterogeneous reactions of methacrolein and methyl vinyl ketone: kinetics and mechanisms of uptake and ozonolysis on silicon dioxide. J. Geophys. Res.-Atmos. 113(D22) (2008). doi:10.1029/2007jd009754
Chiavarino, B., Crestoni, M.E., Fornarini, S., Dopfer, O., Lemaire, J., Maitre, P.: Ir spectroscopic features of gaseous C7h7o+ ions: benzylium versus tropylium ion structures. J. Phys. Chem. A 110(30), 9352–9360 (2006). doi:10.1021/Jp0628380
Claeys, M., Wang, W., Ion, A.C., Kourtchev, I., Gelencser, A., Maenhaut, W.: Formation of secondary organic aerosols from isoprene and its gas-phase oxidation products through reaction with hydrogen peroxide. Atmos. Environ. 38(25), 4093–4098 (2004). doi:10.1016/j.atmosenv.2004.06.001
de Gouw, J.A., Middlebrook, A.M., Warneke, C., Goldan, P.D., Kuster, W.C., Roberts, J.M., Fehsenfeld, F.C., Worsnop, D.R., Canagaratna, M.R., Pszenny, A.A.P., Keene, W.C., Marchewka, M., Bertman, S.B., Bates, T.S.: Budget of organic carbon in a polluted atmosphere: results from the new England air quality study in 2002. J. Geophys. Res.-Atmos. 110(D16) (2005). doi:10.1029/2004jd005623
Drexler, E.J., Field, K.W.: Nmr-study of keto-enol tautomerism in beta-dicarbonyl compounds. J. Chem. Educ. 53(6), 392–393 (1976)
Duncan, J.L., Schindler, L.R., Roberts, J.T.: A new sulfate-mediated reaction: conversion of acetone to trimethylbenzene in the presence of liquid sulfuric acid. Geophys. Res. Lett. 25(5), 631–634 (1998). doi:10.1029/98gl00250
Duncan, J.L., Schindler, L.R., Roberts, J.T.: Chemistry at and near the surface of liquid sulfuric acid: a kinetic, thermodynamic, and mechanistic analysis of heterogeneous reactions of acetone. J. Phys. Chem. B 103(34), 7247–7259 (1999). doi:10.1021/jp991322w
Ervens, B., Turpin, B.J., Weber, R.J.: Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): a review of laboratory, field and model studies. Atmos. Chem. Phys. 11(21), 11069–11102 (2011). doi:10.5194/acp-11-11069-2011
Galloway, M.M., Chhabra, P.S., Chan, A.W.H., Surratt, J.D., Flagan, R.C., Seinfeld, J.H., Keutsch, F.N.: Glyoxal uptake on ammonium sulphate seed aerosol: reaction products and reversibility of uptake under dark and irradiated conditions. Atmos. Chem. Phys. 9(10), 3331–3345 (2009)
Heald, C.L., Jacob, D.J., Park, R.J., Russell, L.M., Huebert, B.J., Seinfeld, J.H., Liao, H., Weber, R.J.: A large organic aerosol source in the free troposphere missing from current models. Geophys. Res. Lett. 32(18) (2005). doi:10.1029/2005gl023831
Hennigan, C.J., Bergin, M.H., Dibb, J.E., Weber, R.J.: Enhanced secondary organic aerosol formation due to water uptake by fine particles. Geophys. Res. Lett. 35(18) (2008). doi:10.1029/2008gl035046
Imamura, T., Akiyoshi, H.: Uptake of acetone into sulfuric-acid solutions. Geophys. Res. Lett. 27(9), 1419–1422 (2000). doi:10.1029/1999gl011137
Iraci, L.T., Tolbert, M.A.: Heterogeneous interaction of formaldehyde with cold sulfuric acid: implications for the upper troposphere and lower stratosphere. J. Geophys. Res.-Atmos. 102(D13), 16099–16107 (1997). doi:10.1029/97jd01259
Jang, M.S., Kamens, R.M.: Atmospheric secondary aerosol formation by heterogeneous reactions of aldehydes in the presence of a sulfuric acid aerosol catalyst. Environ. Sci. Technol. 35(24), 4758–4766 (2001). doi:10.1021/Es010790s
Jang, M.S., Czoschke, N.M., Lee, S., Kamens, R.M.: Heterogeneous atmospheric aerosol production by acid-catalyzed particle-phase reactions. Science 298(5594), 814–817 (2002)
Jang, M., Lee, S., Kamens, R.M.: Organic aerosol growth by acid-catalyzed heterogeneous reactions of octanal in a flow reactor. Atmos. Environ. 37(15), 2125–2138 (2003a). doi:10.1016/S1352-2310(03)00077-3
Jang, M.S., Carroll, B., Chandramouli, B., Kamens, R.M.: Particle growth by acid-catalyzed heterogeneous reactions of organic carbonyls on preexisting aerosols. Environ. Sci. Technol. 37(17), 3828–3837 (2003b). doi:10.1021/Es021006u
Jang, M., Czoschke, N.M., Northcross, A.L.: Atmospheric organic aerosol production by heterogeneous acid-catalyzed reactions. Chemphyschem 5(11), 1647–1661 (2004). doi:10.1002/cphc.200301077
Jaoui, M., Kleindienst, T.E., Lewandowski, M., Edney, E.O.: Identification and quantification of aerosol polar oxygenated compounds bearing carboxylic or hydroxyl groups. 1. Method development. Anal. Chem. 76(16), 4765–4778 (2004). doi:10.1021/Ac049919h
Jaoui, M., Corse, E., Kleindienst, T.E., Offenberg, J.H., Lewandowski, M., Edney, E.O.: Analysis of secondary organic aerosol compounds from the photooxidation of D-limonene in the presence of NOx and their detection in ambient Pm2.5. Environ. Sci. Technol. 40(12), 3819–3828 (2006). doi:10.1021/Es052566z
Jayne, J.T., Worsnop, D.R., Kolb, C.E., Swartz, E., Davidovits, P.: Uptake of gas-phase formaldehyde by aqueous acid surfaces. J. Phys. Chem. 100(19), 8015–8022 (1996)
Kroll, J.H., Seinfeld, J.H.: Chemistry of secondary organic aerosol: formation and evolution of low-volatility organics in the atmosphere. Atmos. Environ. 42(16), 3593–3624 (2008). doi:10.1016/j.atmosenv.2008.01.003
Kroll, J.H., Ng, N.L., Murphy, S.M., Varutbangkul, V., Flagan, R.C., Seinfeld, J.H.: Chamber studies of secondary organic aerosol growth by reactive uptake of simple carbonyl compounds. J. Geophys. Res.-Atmos. 110(D23) (2005). doi:10.1029/2005jd006004
Li, Y.J., Lee, A.K.Y., Lau, A.P.S., Chan, C.K.: Accretion reactions of octanal catalyzed by sulfuric acid: product identification, reaction pathways, and atmospheric implications. Environ. Sci. Technol. 42(19), 7138–7145 (2008). doi:10.1021/Es7031373
Li, Y.J., Cheong, G.Y.L., Lau, A.P.S., Chan, C.K.: Acid-catalyzed condensed-phase reactions of limonene and terpineol and their impacts on gas-to-particle partitioning in the formation of organic aerosols. Environ. Sci. Technol. 44(14), 5483–5489 (2010). doi:10.1021/Es101231m
Li, Y.J., Chen, Q., Guzman, M.I., Chan, C.K., Martin, S.T.: Second-generation products contribute substantially to the particle-phase organic material produced by beta-caryophyllene ozonolysis. Atmos. Chem. Phys. 11(1), 121–132 (2011). doi:10.5194/acp-11-121-2011
Liggio, J., Li, S.M.: Reversible and irreversible processing of biogenic olefins on acidic aerosols. Atmos. Chem. Phys. 8(7), 2039–2055 (2008)
Liggio, J., Li, S.M., McLaren, R.: Heterogeneous reactions of glyoxal on particulate matter: identification of acetals and sulfate esters. Environ. Sci. Technol. 39(6), 1532–1541 (2005)
Liggio, J., Li, S.M.: Organosulfate formation during the uptake of pinonaldehyde on acidic sulfate aerosols. Geophys. Res. Lett. 33(13) (2006). doi:10.1029/2006gl026079
Lim, Y.B., Tan, Y., Perri, M.J., Seitzinger, S.P., Turpin, B.J.: Aqueous chemistry and its role in secondary organic aerosol (SOA) formation. Atmos. Chem. Phys. 10(21), 10521–10539 (2010). doi:10.5194/acp-10-10521-2010
Liu, P., Zhang, Y.: A computationally-efficient secondary organic aerosol module for three-dimensional air quality models. Atmos Chem. Phys. 8(14), 3985–3998 (2008)
Martin-Reviejo, M., Wirtz, K.: Is benzene a precursor for secondary organic aerosol? Environ. Sci. Technol. 39(4), 1045–1054 (2005). doi:10.1021/Es049802a
McLafferty, F.W., Turecek, F.: Interpretation of Mass Spectra, 4th edn. University Science Books, Mill Valley (1993)
Michelsen, R.R., Ashbourn, S.F.M., Iraci, L.T.: Dissolution, speciation, and reaction of acetaldehyde in cold sulfuric acid. J. Geophys. Res.-Atmos. 109(D23) (2004). doi:10.1029/2004jd005041
Noziere, B., Voisin, D., Longfellow, C.A., Friedli, H., Henry, B.E., Hanson, D.R.: The uptake of methyl vinyl ketone, methacrolein, and 2-methyl-3-butene-2-ol onto sulfuric acid solutions. J. Phys. Chem. A 110(7), 2387–2395 (2006). doi:10.1021/Jp055899
Noziere, B., Ekstrom, S., Alsberg, T., Holmstrom, S.: Radical-initiated formation of organosulfates and surfactants in atmospheric aerosols. Geophys. Res. Lett. 37 (2010). doi:10.1029/2009gl041683
Pathak, R.K., Louie, P.K.K., Chan, C.K.: Characteristics of aerosol acidity in Hong Kong. Atmos. Environ. 38(19), 2965–2974 (2004). doi:10.1016/j.atmosenv.2004.02.044
Pathak, R.K., Wu, W.S., Wang, T.: Summertime Pm2.5 ionic species in four major cities of China: nitrate formation in an ammonia-deficient atmosphere. Atmos. Chem. Phys. 9(5), 1711–1722 (2009)
Perri, M.J., Lim, Y.B., Seitzinger, S.P., Turpin, B.J.: Organosulfates from glycolaldehyde in aqueous aerosols and clouds: laboratory studies. Atmos. Environ. 44(21–22), 2658–2664 (2010). doi:10.1016/j.atmosenv.2010.03.031
Pun, B.K., Seigneur, C.: Investigative modeling of new pathways for secondary organic aerosol formation. Atmos. Chem. Phys. 7(9), 2199–2216 (2007)
Pun, B.K., Griffin, R.J., Seigneur, C., Seinfeld, J.H.: Secondary organic aerosol—2. Thermodynamic model for gas/particle partitioning of molecular constituents. J. Geophys. Res.-Atmos. 107(D17) (2002). doi:10.1029/2001jd000542
Rudich, Y., Donahue, N.M., Mentel, T.F.: Aging of organic aerosol: bridging the gap between laboratory and field studies. Annu. Rev. Phys. Chem. 58, 321–352 (2007). doi:10.1146/annurev.physchem.58.032806.104432
Seinfeld, J.H., Erdakos, G.B., Asher, W.E., Pankow, J.F.: Modeling the formation of secondary organic aerosol (SOA). 2. The predicted effects of relative humidity on aerosol formation in the alpha-pinene-, beta-pinene-, sabinene-, delta(3)-carene-, and cyclohexene-ozone systems. Environ. Sci. Technol. 35(9), 1806–1817 (2001). doi:10.1021/Es001765+
Stone, E.A., Yang, L.M., Yu, L.Y.E., Rupakheti, M.: Characterization of organosulfates in atmospheric aerosols at four Asian locations. Atmos. Environ. 47, 323–329 (2012). doi:10.1016/j.atmosenv.2011.10.058
Surratt, J.D., Kroll, J.H., Kleindienst, T.E., Edney, E.O., Claeys, M., Sorooshian, A., Ng, N.L., Offenberg, J.H., Lewandowski, M., Jaoui, M., Flagan, R.C., Seinfeld, J.H.: Evidence for organosulfates in secondary organic aerosol. Environ. Sci. Technol. 41(2), 517–527 (2007). doi:10.1021/es062081q
Surratt, J.D., Gomez-Gonzalez, Y., Chan, A.W.H., Vermeylen, R., Shahgholi, M., Kleindienst, T.E., Edney, E.O., Offenberg, J.H., Lewandowski, M., Jaoui, M., Maenhaut, W., Claeys, M., Flagan, R.C., Seinfeld, J.H.: Organosulfate formation in biogenic secondary organic aerosol. J. Phys. Chem. A 112(36), 8345–8378 (2008). doi:10.1021/Jp802310p
Surratt, J.D., Chan, A.W.H., Eddingsaas, N.C., Chan, M.N., Loza, C.L., Kwan, A.J., Hersey, S.P., Flagan, R.C., Wennberg, P.O., Seinfeld, J.H.: Reactive intermediates revealed in secondary organic aerosol formation from isoprene. Proc. Natl. Acad. Sci. U.S.A. 107(15), 6640–6645 (2010). doi:10.1073/pnas.0911114107
Szmigielski, R., Surratt, J.D., Vermeylen, R., Szmigielska, K., Kroll, J.H., Ng, N.L., Murphy, S.M., Sorooshian, A., Seinfeld, J.H., Claeys, M.: Characterization of 2-methylglyceric acid oligomers in secondary organic aerosol formed from the photooxidation of isoprene using trimethylsilylation and gas chromatography/ion trap mass spectrometry. J. Mass Spectrom. 42(1), 101–116 (2007). doi:10.1002/Jms.1146
Volkamer, R., Jimenez, J.L., San Martini, F., Dzepina, K., Zhang, Q., Salcedo, D., Molina, L.T., Worsnop, D.R., Molina, M.J.: Secondary organic aerosol formation from anthropogenic air pollution: rapid and higher than expected. Geophys. Res. Lett. 33(17) (2006). doi:10.1029/2006gl026899
Volkamer, R., Martini, F.S., Molina, L.T., Salcedo, D., Jimenez, J.L., Molina, M.J.: A missing sink for gas-phase glyoxal in Mexico City: formation of secondary organic aerosol. Geophys. Res. Lett. 34(19) (2007). doi:10.1029/2007gl030752
Volkamer, R., Ziemann, P.J., Molina, M.J.: Secondary organic aerosol formation from acetylene (C2h2): seed effect on SOA yields due to organic photochemistry in the aerosol aqueous phase. Atmos. Chem. Phys. 9(6), 1907–1928 (2009)
Wang, D.W., Guo, H., Chan, C.K.: Measuring ambient acidic ultrafine particles using iron nanofilm detectors: method development. Aerosol Sci. Technol. 46(5), 521–532 (2012). doi:10.1080/02786826.2011.643258
Wexler, A.S., Clegg, S.L.: Atmospheric aerosol models for systems including the ions H+, Nh4+, Na+, So42−, No3−,Cl−, Br−, and H2o. J. Geophys. Res.-Atmos. 107(D14) (2002). doi:10.1029/2001jd000451
Yu, J.Z., Flagan, R.C., Seinfeld, J.H.: Identification of products containing –Cooh, –OH, and –C=O in atmospheric oxidation of hydrocarbons. Environ. Sci. Technol. 32(16), 2357–2370 (1998)
Zhang, Q., Jimenez, J.L., Worsnop, D.R., Canagaratna, M.: A case study of urban particle acidity and its influence on secondary organic aerosol. Environ. Sci. Technol. 41(9), 3213–3219 (2007). doi:10.1021/Es061812j
Acknowledgement
This work was supported by the Research Grants Council of Hong Kong (Project No. 610909) and by U.S. National Science Foundation grant AGS-1057183.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 438 kb)
Rights and permissions
About this article
Cite this article
Chan, K.M., Huang, D.D., Li, Y.J. et al. Oligomeric products and formation mechanisms from acid-catalyzed reactions of methyl vinyl ketone on acidic sulfate particles. J Atmos Chem 70, 1–18 (2013). https://doi.org/10.1007/s10874-013-9248-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-013-9248-7