Abstract
The molecular structure of (2,6-iPr2C6H3)N(quin)2 (1) and both the methanol and toluene solvates of its copper complex [Cu{(2,6-iPr2C6H3)N(quin)2}2]BF4, 2·MeOH and 2.2(C6H5Me), respectively, have been determined. The quinolyl rings in 1 adopt anti-syn (CAr–N–C–Nquin) conformation as a result of π–π stacking. The cation in 2·MeOH crystallizes on a C2 axis, while the cation in 2·2(C6H5Me) is crystallographically independent. As a result of intramolecular π–π stacking there are significant changes within the coordination geometry about the copper centers between the two solvates, suggesting that the coordination around copper is supple. Crystal data: 1 group P21/c, a = 8.614(1), b = 16.137(3), c = 17.601(4) Å, β = 93.32(3)°, V = 2,442.5(9) Å3, Z = 4, R = 0.0544, wR 2 = 0.1340. 2·MeOH group P3221, a = 13.254(1), b = 13.254(1), c = 27.214(5) Å, V = 4,140(1) Å3, Z = 3, R = 0.0392, wR 2 = 0.0917. 2.2(C6H5Me) group P1, a = 11.677(2), b = 16.261(3), c = 17.077(3) Å, α = 93.63(3), β = 97.30(3), γ = 96.26(3)°, V = 3,187(1) Å3, Z = 4, R = 0.0526, wR 2 = 0.1221.
Graphical Abstract
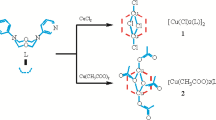
The molecular structure of (2,6-iPr2C6H3)N(quin)2 and both the methanol and toluene solvates of its copper complex [Cu{(2,6-iPr2C6H3)N(quin)2}2]BF4 have been determined. The presence of inter- and intra-molecular π–π interactions dominates the conformations of the quinolyl substituents.







Similar content being viewed by others
References
Thompson JS, Whitney JF (1984) Inorg Chem 23:2813
Allen JJ, Barron AR (2009) Dalton Trans 878
Allen JJ, Hamilton CE, Barron AR (submitted for publication)
Allen JJ, Barron AR (2008) J Chem Cryst 38:879
Schareina T, Hillebrand G, Fuhrmann H, Kempe R (2001) Eur J Inorg Chem 9:2421
Polyakova IN, Starikova ZA, Trunov VK, Parusnikov BV, Krasavin IA (1980) Kristallografiya 25:495
Hathaway BJH, Holah DG, Postlethwaite JD (1961) Chem Comm 3215
Ruhl S, Bolte M (2000) Z Kristallogr 215:499
Bruker AXS (1999) SMART v5.050; Bruker molecular analysis research tool. Bruker AXS, Madison, WI
Bruker AXS (1999) SAINT v6.06. Integration software for single crystal data. Bruker AXS, Madison, WI
Sheldrick GM (2008) SADABS, Bruker area detector absorption corrections. Bruker AXS, Madison, WI, based on method described in: Blessing RH. Acta Crystallogr A51, 33–38
Sheldrick GM (2008) Acta Cryst A64:112
Ibers J, Hamilton WC (1984) International tables for X-ray crystallography, vol 4. 1974, Kynoch Press, Birmingham
Sheldrick GM (2008) SHELXTL v6.14; The complete software package for single crystal structure determination. Bruker AXS, Madison, WI
Spek AL (2008) PLATON, molecular geometry program. Utrecht, Holland, University of Ulrecht
Spek AL (2003) J Appl Cryst 36:7
Flack HD, Bernardinelli G (2000) J Appl Cryst 33:1143
Clegg W (2003) Acta Cryst E59:e2
Allen JJ, Hamilton CE, Barron AR (2008) J Chem Cryst 38:873
Yang J-S, Lin Y-D, Lin Y-H, Liao F-L (2004) J Org Chem 69:3517
Sumby C, Steel P (2007) Inorg Chim Acta 360:2100
Polyakova IN, Starikova ZA, Parusnikov BV, Krasavin IA (1985) Kristallografiya 30:1010
CRC handbook of chemistry and physics, 60th edn. CRC Press, Boca Raton, FL, 1980, D-194
Acknowledgments
Financial support for this work is provided by Trans Ionics Corporation. The Robert A. Welch Foundation is acknowledged for funding of the Texas Center for Crystallography at Rice University. We acknowledge Dr. Robert C. Schucker and Michael F. Lynch for useful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allen, J.J., Hamilton, C.E. & Barron, A.R. Synthesis and Structural Characterization of (2,6-iPr2C6H3)N(quin)2 and [Cu{(2,6-iPr2C6H3)N(quin)2}2]BF4 . J Chem Crystallogr 40, 130–136 (2010). https://doi.org/10.1007/s10870-009-9615-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-009-9615-z