Abstract
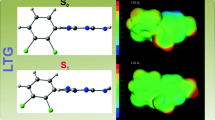
The X-ray crystal structures of two lamotrigine derivatives (I) 2-methyl, 3-amino, 5-imino-6-(2, 3-dichlorophenyl)-1,2,4-triazine, C10H9Cl2N5, as the hemi hydrate and (II) 2-methyl,3,5-diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine, C10H10Cl2N5, as the isethionate-water solvate, have been carried out at liquid nitrogen temperature. A detailed comparison of the two structures is given. Both are monoclinic and centrosymmetric, with (I) in space group C2/c, and (II) in space group P21/n. For (I) the unit cell dimensions are a = 19.5466(10), b = 7.5483(4), c = 15.7861(8) Å, β = 91.458(3)°, volume = 2328.4(2) Å3, Z = 8, density = 1.590 Mg/m3; for (II). For (II) the unit cell dimensions are a = 6.0566(2), b = 11.0084(4) c = 23.9973(9) Å, β = 92.587(3)°, volume = 1598.35(10) Å3, Z = 4, density = 1.597 Mg/m3. For (I) final R indices [I > 2sigma(I)] are R1 = 0.0356, wR2 = 0.0782 and R indices (all data) are R1 = 0.0424, wR2 = 0.0817. For (II) final R indices [I > 2sigma(I)] are R1 = 0.0380, wR2 = 0.0871 and R indices (all data) R1 = 0.0558, wR2 = 0.0949. Both structures have a molecule of water of crystallization and (II) also includes a solvated CH3SO3. Comparisons are made between the two structures. Structure (I) is very unusual in having a = NH group at position C5′ on the triazine ring. No other examples of this particular substitution, which is usually −NH2, have been reported.
Index Abstract
Rex A. Palmer, Brian S. Potter, Michael J Leach and Babur Z. Chowdhry
The crystal structures of (I) 2-methyl,3-amino, 5-imino-6-(2, 3-dichlorophenyl)-1, 2, 4-triazine, water solvate and (II) 2-methyl,3, 5-diamino-6-(2, 3-dichlorophenyl)-1, 2, 4-triazine isethionate water solvate are presented. The relative orientation of the two rings is shown to vary. Lamotrigine and analogues have been investigated for some time for their effects on the central nervous system. For example both lamotrigine and 5-(2,3,5-trichlorophenyl)-2,4-diaminopyrimidine (code name BW 1003C87) are voltage-gated sodium channel blockers as well as blocking the release of the neurotransmitter glutamate [D. R. Riddall, M. J. Leach, J. Garthwaite, Mol. Pharmacol. 2006, 69 (1), 278.3], BW10003C87 (like lamotrigine) has been shown to exhibit excitatory amino acid antagonist activity similar to that of three conventional antiepileptic drugs phenytoin, carbamazepine and phenobarbital [R. Lingamaneni, H. C. Hemmings Jr., Epilepsy Res. 1993, 15, 101.]. BW 1003C87 has also been shown [B. S. Meldrum, J. H. Swan, M. J. Leach, M. H. Millan, R. Gwinn, K. Kadota, S. H Graham, J. Chen, R. P. Simon , Brain Res., 1992, 593, 1.] to reduce the release of glutamate evoked by veratrine in brain tissue, providing a therapeutic approach in both cerebral ischemia and epilepsy. This is one of a series of papers on the structures of lamotrigine analogues.





Similar content being viewed by others
References
Janes RW, Palmer RA (1989) Acta Cryst C45:129
Palmer RA, Potter BS, Leach MJ, Chowdhry BZ (2007) J Chem Crystallogr 37:771
Riddall DR, Leach MJ, Garthwaite J (2006) Mol Pharmacol 69(1):278
Lingamaneni R, Hemmings HC Jr (1993) Epilepsy Res 15:101
Meldrum BS, Swan JH, Leach MJ, Millan MH, Gwinn R, Kadota K, Graham SH, Chen J, Simon RP (1992) Brain Res 593:1
Barnes CL (1997) ORTEP-3 for Windows–a version of ORTEP-III with a Graphical User Interface (GUI). J Appl Cryst 30:568. [Based on ORTEP-III (v 1.0.3) by Johnson CK, Burnett MN]
Merrit EA, Bacon DJ (1997) Raster 3D Graphics version 2.7c. Meth Enzymol. 277:505 [Implemented in WinGX (qv) and generated by Ortep-3 for Windows]
Ladd MFC, Palmer RA (2003) Structure Determination by X-ray Crystallography, 4th edn. Klewer-Plenum, NY, p 503
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M (2006) J van de Streek J Appl Cryst 39:453
Hooft R, Nonius BV (1998) COLLECT: X-ray Data collection and processing software user interface
Cosier J, Glazer AM (1986) J Appl Cryst 19:105
Otwinowski Z, Minor W (1997) Meth Enzymol 276:307; In: Carter CW Jr, Sweet RM (eds) Macromolecular crystallography. Academic Press, New York
Blessing RH (1995) Acta Cryst A51:33
R.H. Blessing (1997) J Appl Cryst 30:421
Sheldrick GM (1996) SHELXS-86. Program for Crystal Structure Determination. University of Göttingen, Germany
Sheldrick GM (1997) SHELXL97. Program for Crystal Structure Refinement. University of Göttingen, Germany
Farrugia LJ, Win GX (1999) J Appl Cryst 32:837
Spek AL (1990) Acta Crystallogr A46:C34
Palmer RA, Potter BS, Leach MJ, Chowdhry BZ (2007) J Chem Cryst (Accepted)
Potter BS, Palmer RA, Withnall R, Leach MJ, Chowdhry BZ (1999) J Chem Cryst 29(6):701
Janes RW, Palmer RA (1989) Acta Cryst C45:129
Janes RW, Palmer RA (1995) Acta Cryst C51:440
Janes RW, Palmer RA (1995) Acta Cryst C51:685
Janes RW, Palmer RA (1996) Acta Cryst C52:2627
Janes RW (1999) J Chem Cryst 29(2):163
Janes RW, Palmer RA (1995) J Mol Struct (Theochem) 339:95
Acknowledgments
We thank Dr P. Barraclough (University of Greenwich) for the synthesis and provision of samples of (I) and (II). Low temperature X-ray intensity data were collected on the EPSRC single crystal X-ray data facility at Southampton University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Palmer, R.A., Potter, B.S., Leach, M.J. et al. Low Temperature X-ray Crystallographic Structures of Two Lamotrigine Analogues: (I) 2-Methyl,3-amino, 5-imino-6-(2,3-dichlorophenyl)-1,2,4-triazine Water Solvate and (II) 2-Methyl,3,5-diamino-6-(2,3-dichlorophenyl)-1,2,4-triazine Isethionate, Hemi-hydrate. J Chem Crystallogr 38, 255–260 (2008). https://doi.org/10.1007/s10870-008-9318-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-008-9318-x