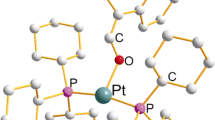
We wish to report the crystal structure for the heteroleptic platinum(II) complex with the crown thioether 1,4,7-trithiacyclononane (9S3) and the diimine ligand 3,4,7,8-tetramethyl-1,10-phenanthroline (tmphen), [Pt(9S3)(tmphen)](PF6)2 (1). The Pt(II) center is surrounded by a cis-S2N2 ligand environment formed by the bidentate diimine and two of the three sulfur atoms from the 9S3 ligand. These two sulfurs are positioned 2.2665(10) and 2.2612(11) Å from the Pt(II), but the third sulfur shows a long distance interaction at 2.8849(13) Å to form an elongated square pyramidal structure. An examination of the structure reveals π–π-intermolecular stacking of the phen groups with alternating distances of 3.53 and 3.62 Å between the planes of the phen ligands. A detailed survey of 12 other divalent Pt(II) and Pd(II) diimine complexes of 9S3 reveals three different stacking motifs involving π–π interactions in the solid state. Crystal Data for (1): P-1, a = 10.868(2) Å, b = 11.292(2) Å, c = 11.513(2) Å, α = 93.76(3)°, β = 90.82(3)°, γ = 92.28(3)°, V = 1408.5(5) Å3, Z = 2.




Similar content being viewed by others
Notes
While the anions are the same for this series of cation structures, the data collection temperatures for the structures in this series varies considerably (150–303 K), precluding a more detailed comparison of π–π interaction distances.
References
(a) Blake, A.J.; Schröder, M. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Vol. 35, Academic Press: New York, 1990, p. 2; (b) Cooper, S.R. Acc. Chem. Res. 1988, 21, 141; (c) Cooper, S.; Rawle, S.C. Struct. Bonding (Berlin) 1990, 72, 1; (d) Schröder, M. Pure Appl. Chem. 1988, 60, 517.
(a) Blake, A.J.; Gould, R.O.; Holder, A.J.; Hyde, T.I.; Lavery, A.J.; Odulate, M.O.; Schröder, M. J. Chem. Soc. Chem. Commun. 1987, 118; (b) Grant, G.J.; Sanders, K.A.; Setzer, W.N.; VanDerveer, D.G. Inorg. Chem. 1991, 30, 4053; (c) Chandrasekhar, S.; McAuley, A. Inorg. Chem. 1992, 31, 2663; (d) Grant, G.J.; Goforth, A.M.; VanDerveer, D.G.; Pennington, W.P. Inorg. Chem. Acta 2004, 357, 2107; (e) Blake, A.J.; Holder, A.J.; Hyde, T.I.; Roberts, Y.V.; Lavery, A.J.; Schröder, M. J. Organometal. Chem. 1987, 323, 261; (f) Blake, A.J.; Crofts, R.D.; Schröder, M. J. Chem. Soc. Dalton Trans. 1993, 2259; (g) Grant, G.J.; Spangler, N.S.; Setzer, W.N.; VanDerveer, D.G. Inorg. Chim. Acta 1996, 246, 41.
The sum of the van der Waals radii for Pt(II) and sulfur is 3.50 Å. In Inorganic Chemistry: Principles of Structure and Reactivity, Huheey, J.E.; Keiter, E.A.; Keiter, R.L., Eds.; 4th Edn. Harper Collins: New York, 1993, p. 292.
Grant, G.J.; Chen, W.; Goforth, A.M.; Baucom, C.L.; Patel, K.; Repovic, P.; VanDerveer, D.G.; Pennington, W.T. Eur. J. Inorg. Chem. 2005, 479.
Hambley, T.W. Inorg. Chem. 1998, 37, 3767.
Grant, G.J.; Carter, S.M.; Russell, A.L.; Poullaos, I.M.; VanDerveer, D.G. J. Organometal. Chem. 2001, 683, 637– 639.
(a) Grant, G.J.; Brandow, C.G.; Galas, D.F.; Davis, J.P.; Pennington, W.T.; Zubkowski, J.D.; Valente, E.J. Polyhedron 2001, 20, 3333; (b) Grant, G.J.; Poullaos, I.M.; Galas, D.F.; VanDerveer, D.G.; Zubkowski, J.D.; Valente, E.J. Inorg. Chem. 2001, 40, 564; (c) Grant, G.J., Galas, D.F., VanDerveer, D.G. Polyhedron 2002, 21, 879; (d) Grant, G.J.; Galas, D.F.; Poullaos, I.M.; Carter, S.M.; VanDerveer, D.G. J. Chem. Soc., Dalton Trans. 2002, 15, 2973; (e) Grant, G.J.; Pool, J.P.; VanDerveer, D.G. J. Chem. Soc., Dalton Trans. 2003, 3981; (f) Bennett, M.A.; Canty, A.J.; Felixberger, J.K.; Rendina, L.M.; Sunderland, C.; Willis, A.C. Inorg. Chem. 1993, 32, 1951; (g) Bennett, M.A.; Felixberger, J.K.; Willis, A.C. Gazz. Chim. Ital. 1993, 123, 405; Lee, G.-H.; Acta Cryst. 1998, C54, 906.
Grant, G.J.; Poullaos, I.M.; Galas, D.F.; VanDerveer, D.G.; Zubkowski, J.D.; Valente, E.J. Inorg. Chem. 2001, 40, 564.
Grant, G.J., Galas, D.F., VanDerveer, D.G. Polyhedron 2002, 21, 879.
Grant, G.J.; Galas, D.F.; Poullaos, I.M.; Carter, S.M.; VanDerveer, D.G. J. Chem. Soc., Dalton Trans. 2002, 15, 2973.
Grant, G.J.; Pool, J.P.; VanDerveer, D.G. J. Chem. Soc., Dalton Trans. 2003, 3981.
Bennett, M.A.; Canty, A.J.; Felixberger, J.K.; Rendina, L.M.; Sunderland, C.; Willis, A.C. Inorg. Chem. 1993, 32, 1951.
Bennett, M.A.; Felixberger, J.K.; Willis, A.C. Gazz. Chim. Ital. 1993, 123, 405.
Lee, G.-H.; Acta Cryst. 1998, C54, 906.
Blake, A.J.; Roberts, Y.V.; Schröder, M. J. Chem. Soc. Dalton 1996, 1885.
Grant, G.J.; Patel, K.N.; Helm, M.L.; Mehne, L.F.; Klinger, D.W.; VanDerveer, D.G. Polyhedron 2004, 23, 1361.
Green, T.W.; Lieberman, R.; Mitchell, N.; Krause Bauer, J.A.; Connick, W.B. Inorg. Chem. 2005, 44(6), 1955.
(a) CrystalClear; Rigaku/MSC, The Woodlands, TX, USA, 1999; (b) Jacobson, R.A. REQAB, subroutine of CrystalClear; Rigaku/MSC, The Woodlands, TX, USA, 1999.
SHELXTL 5.1 1998–1999, Bruker AXS, Madison, WI, USA.
Mercury v 1.3. Cambridge Crytallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ. U.K.
Nikol, H.; Bürgi, H.-B.; Hardcastle, K.I.; Gray, H.B. Inorg. Chem. 1995, 34, 6319.
Janiak, C. J. Chem. Soc. Dalton Trans. 2000, 3885.
van der Waals radii (C = 1.70 Å, N = 1.55 Å) are taken from Bondi, A. J. Phys. Chem. 1964, 68, 441.
(a) Cambridge Structural Database v 5.26 November 2004, Cambridge Crytallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ. U.K; (b) The search was limited to L being a C2 symmetric diimine. One related additional structure outside our search parameters is [Pd(9S3)(5-NO2-phen)](PF6)2·CH3NO2 (CCDC code SAVGUR). Blake, A.J.; Champness, N.R.; Li, W.-S.; Schröder, M. Acta Cryst. 1998, C54, IUC9800071.
Acknowledgments
This research was generously supported by grants from the Petroleum Research Fund, administered by the American Chemical Society, the Grote Chemistry Fund at the University of Tennessee at Chattanooga, the Wheeler Odor Center at the University of Tennessee at Chattanooga and by the National Science Foundation, Research at Undergraduate Institutions Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary material
Crystallographic data for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as CCDC 267337. Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge, CB2 1 EZ UK (Fax: +44-1223-336-033); E-MAIL: deposit@ccdc.cam.ac.uk.
Rights and permissions
About this article
Cite this article
Janzen, D.E., Patel, K., VanDerveer, D.G. et al. π–π Interactions in diimine Pt(II) complexes with thiacrown ligands: The crystal structure of (1,4,7-trithiacyclononane)(3,4,7,8-tetramethyl-1, 10-phenanthroline)platinum(II) hexafluorophosphate. J Chem Crystallogr 36, 83–91 (2006). https://doi.org/10.1007/s10870-005-9002-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-005-9002-3