Abstract
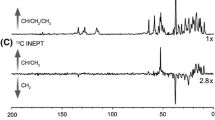
The second isoform of the human voltage dependent anion channel (VDAC2) is a mitochondrial porin that translocates calcium and other metabolites across the outer mitochondrial membrane. VDAC2 has been implicated in cardioprotection and plays a critical role in a unique apoptotic pathway in tumor cells. Despite its medical importance, there have been few biophysical studies of VDAC2 in large part due to the difficulty of obtaining homogeneous preparations of the protein for spectroscopic characterization. Here we present high resolution magic angle spinning nuclear magnetic resonance (NMR) data obtained from homogeneous preparation of human VDAC2 in 2D crystalline lipid bilayers. The excellent resolution in the spectra permit several sequence-specific assignments of the signals for a large portion of the VDAC2 N-terminus and several other residues in two- and three-dimensional heteronuclear correlation experiments. The first 12 residues appear to be dynamic, are not visible in cross polarization experiments, and they are not sufficiently mobile on very fast timescales to be visible in 13C INEPT experiments. A comparison of the NMR spectra of VDAC2 and VDAC1 obtained from highly similar preparations demonstrates that the spectral quality, line shapes and peak dispersion exhibited by the two proteins are nearly identical. This suggests an overall similar dynamic behavior and conformational homogeneity, which is in contrast to two earlier reports that suggested an inherent conformational heterogeneity of VDAC2 in membranes. The current data suggest that the sample preparation and spectroscopic methods are likely applicable to studying other human membrane porins, including human VDAC3, which has not yet been structurally characterized.





Similar content being viewed by others
References
Andreas LB, Eddy MT, Chou JJ, Griffin RG (2012) Magic-angle-spinning NMR of the drug resistant S31N M2 proton transporter from influenza A. J Am Chem Soc 134:7215–7218
Andreas LB, Barnes AB, Corzilius B, Chou JJ, Miller EA, Caporini M, Rosay M, Griffin RG (2013) Dynamic nuclear polarization study of inhibitor binding to the M2 proton transporter from influenza A. Biochemistry 52:2774–2782
Andreas LB, Reese M, Eddy MT, Gelev V, Ni QZ, Miller EA, Emsley L, Pintacuda G, Chou JJ, Griffin RG (2015) Structure and function of the influenza A M218-60 dimer of dimers. J Am Chem Soc 137:14877–14886
Andronesi OC, Becker S, Seidel K, Heise H, Young HS, Baldus M (2005) Determination of membrane protein structure and dynamics by magic-angle-spinning solid-state NMR spectroscopy. J Am Chem Soc 127:12965–12974
Bajaj VS, Mak-Jurkauskas ML, Belenky M, Herzfeld J, Griffin RG (2009) Functional and shunt states of bacteriorhodopsin resolved by 250-GHz dynamic nuclear polarization-enhanced solid-state NMR Proc. Natl. Acad. Sci. 106:9244–9249
Bauer AJ, Gieschler S, Lemberg KM, McDermott AE, Stockwell BR (2011) Functional model of metabolite gating by human voltage-dependent anion channel 2. Biochemistry 50:3408–3410
Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K (2008) Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA 105:15370–15375
Bennett AE, Griffin RG, Ok JH, Vega S (1992) Chemical shift correlation spectroscopy in rotating solids: Radio frequency-driven dipolar recoupling and longitudinal exchange. J Chem Phys 96:8624–8627
Bennett AE, Rienstra CM, Griffiths JM, Zhen, W, Lansbury PT Jr, Griffin RG (1998) Homonuclear radio frequency-driven recoupling in rotating solids. J Chem Phys 108:9463–9479
Blachly-Dyson E, Peng S, Colombini M, Forte M (1990) Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications. Science 247:1233–1236
Blachly-Dyson E, Zambronicz EB, Yu WH, Adams V, McCabe ER, Adelman J, Colombini M, Forte M (1993) Cloning and functional expression in yeast of two human isoforms of the outer mitochondrial membrane channel, the voltage-dependent anion channel. J Biol Chem 268:1835–1841
Cheng EHY, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301:513–517
Colombini M (1979) A candidate for the permeability pathway of the outer mitochondrial membrane. Nature 279:643–645
Colombini M (2004) VDAC: the channel at the interface between mitochondria and the cytosol. Mol Cell Biochem 256–257:107–115
Colombini M (2012) VDAC structure, selectivity, and dynamics. BBA-Biomembranes 1818:1457–1465
Colombini M (2016) The VDAC channel: molecular basis for selectivity. BBA-Mol Cell Res 1863:2498–2502
Daviso E, Eddy MT, Andreas LB, Griffin RG, Herzfeld J (2013) Efficient resonance assignment of proteins in MAS NMR by simultaneous intra- and inter-residue 3D correlation spectroscopy. J Biomol NMR 55:257–265
Dolder M, Zeth K, Tittmann P, Gross H, Welte W, Wallimann T (1999) Crystallization of the human, mitochondrial voltage-dependent anion-selective channel in the presence of phospholipids. J Struct Biol 127:64–71
Eddy MT, Ong T-C, Clark L, Teijido O, van der Wel PCA, Garces R, Wagner G, Rostovtseva TK, Griffin RG (2012) Lipid dynamics and protein-lipid interactions in 2D crystals formed with the β-barrel integral membrane protein VDAC1. J Am Chem Soc 134:6375–6387
Eddy MT, Andreas L, Teijido O, Su Y, Clark L, Noskov SY, Wagner G, Rostovtseva TK, Griffin RG (2015a) Magic angle spinning nuclear magnetic resonance characterization of voltage-dependent anion channel gating in two-dimensional lipid crystalline bilayers. Biochemistry 54:994–1005
Eddy MT, Su Y, Silvers R, Andreas L, Clark L, Wagner G, Pintacuda G, Emsley L, Griffin RG (2015b) Lipid bilayer-bound conformation of an integral membrane beta barrel protein by multidimensional MAS NMR. J Biomol NMR 61:299–310
Etzkorn M, Martell S, Andronesi OC, Seidel K, Engelhard M, Baldus M (2007) Secondary structure, dynamics, and topology of a seven-helix receptor in native membranes, studied by solid-state NMR spectroscopy. Angew Chem Int Ed 46:459–462
Gattin Z, Schneider R, Laukat Y, Giller K, Maier E, Zweckstetter M, Griesinger C, Benz R, Becker S, Lange A (2015) Solid-state NMR, electrophysiology and molecular dynamics characterization of human VDAC2. J Biomol NMR 61:311–320
Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G (2008) Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 321:1206–1210
Hing AW, Vega S, Schaefer J (1993) Measurement of heteronuclear dipolar coupling by transferred-echo double-resonance NMR. J Magn Reson Ser A 103:151–162
Jaroniec CP, Tounge BA, Rienstra CM, Herzfeld J, Griffin RG (1999) Measurement of C-13-N-15 distances in uniformly C-13 labeled biomolecules: J-decoupled REDOR. J Am Chem Soc 121:10237–10238
Jaroniec CP, Filip C, Griffin RG (2002) 3D TEDOR NMR experiments for the simultaneous measurement of multiple carbon-nitrogen distances in uniformly C-13, N-15- labeled solids. J Am Chem Soc 124:10728–10742
Lauterwasser J, Todt F, Zerbes RM, Nguyen TN, Craigen W, Lazarou M, van der Laan M, Edlich F (2016) The porin VDAC2 is the mitochondrial platform for Bax retrotranslocation. Nature 6:32994
Liu B, Wang Z, Zhang W, Wang X (2009) Expression and localization of voltage-dependent anion channels (VDAC) in human spermatozoa. Biochem Biophys Res Commun 378:366–370
Long JR, Sun BQ, Bowen A, Griffin RG (1994) Molecular dynamics and magic angle spinning NMR. J Am Chem Soc 116:11950–11956
Luca S, Filippov DV, van Boom JH, Oschkinat H, de Groot HJ, Baldus M (2001) Secondary chemical shifts in immobilized peptides and proteins: a qualitative basis for structure refinement under magic angle spinning. J Biomol NMR 20:325–331
Maurya SR, Mahalakshmi R (2016) Mitochondrial VDAC2 and cell homeostasis: highlighting hidden structural features and unique functionalities. Biol Rev 279:25316–25364
Maus DC, Copié V, Sun B, Griffiths JM, Griffin RG, Luo S, Schrock RR, Liu AH, Seidel SW, Davis WM, Grohmann A (1996) A solid-state NMR study of tungsten methyl group dynamics in [W(η 5-C 5Me 5)Me 4][PF 6]. J Am Chem Soc 118:5665–5671
Menzel VA, Cassará MC, Benz R, De Pinto V, Messina A, Cunsolo V, Saletti R, Hinsch KD, Hinsch E (2009) Molecular and functional characterization of VDAC2 purified from mammal spermatozoa. Biosci Rep 29:351–362
Morris GA, Freeman R (1979) Enhancement of nuclear magnetic resonance signals by polarization transfer. J Am Chem Soc 101:760–762
Naghdi S, Hajnóczky G (2016) VDAC2-specific cellular functions and the underlying structure. BBA-Mol Cell Res 1863:2503–2514
Ni QZ, Can TV, Daviso E, Belenky M, Griffin RG, Herzfeld J (2018) Primary transfer step in the light-driven ion pump bacteriorhodopsin: an irreversible U-Turn revealed by dynamic nuclear polarization-enhanced magic angle spinning NMR. J Am Chem Soc 140:4085–4091
Pines A, Gibby MG, Waugh JS (1973) Proton-enhanced NMR of dilute spins in solids. J Chem Phys 59:569–590
Raghavan A, Sheiko T, Graham BH, Craigen WJ (2012) Voltage-dependant anion channels: novel insights into isoform function through genetic models. BBA-Biomembranes 1818:1477–1485
Rienstra CM, Hohwy M, Hong M, Griffin RG (2000) 2D and 3D N-15-C-13-C-13 NMR chemical shift correlation spectroscopy of solids: assignment of MAS spectra of peptides. J Am Chem Soc 122:10979–10990
Rostovtseva TK, Bezrukov SM (1998) ATP transport through a Single mitochondrial channel, VDAC, studied by current fluctuation analysis. Biophys J 74:2365–2373
Rostovtseva T, Colombini M (1996) ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane. J Biol Chem 271:28006–28008
Rostovtseva T, Colombini M (1997) VDAC channels mediate and gate the flow of ATP: implications for the regulation of mitochondrial function. Biophysical J 72:1954–1962
Schneider R, Etzkorn M, Giller K, Daebel V, Eisfeld J, Zweckstetter M, Griesinger C, Becker S, Lange A (2010) The native conformation of the human VDAC1 N terminus. Angew Chem Int Ed Eng 49:1882–1885
Schredelseker J, Paz A, Lopez CJ, Altenbach C, Leung CS, Drexler MK, Chen JN, Hubbell WL, Abramson J (2014) High resolution structure and double electron-electron resonance of the zebrafish voltage-dependent anion channel 2 reveal an oligomeric population. J Biol Chem 289:12566–12577
Stehle J, Scholz F, Löhr F, Reckel S, Roos C, Blum M, Braun M, Glaubitz C, Dötsch V, Wachtveitl J, Schwalbe H (2012) Characterization of the ground state dynamics of proteorhodopsin by NMR and optical spectroscopies. J Biomol NMR 54:401–413
Sun BQ, Rienstra CM, Costa PR, Williamson JR, Griffin RG (1997) 3D 15N-13C-13C chemical shift correlation spectroscopy in rotating solids. J Am Chem Soc 119:8540–8546
Ujwal R, Cascio D, Colletier J-P, Faham S, Zhang J, Toro L, Ping P, Abramson J (2008) The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA 105:17742–17747
Ward ME, Brown LS, Ladizhansky V (2015) Advanced solid-state NMR techniques for characterization of membrane protein structure and dynamics: application to anabaena sensory rhodopsin. J Magn Reson 253:119–128
Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR (2007) RAS–RAF–MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447:865–869
Yoo BC, Fountoulakis M. Cairns N. Lubec G (2001) Changes of voltage-dependent anion-selective channel proteins VDAC1 and VDAC2 brain levels in patients with Alzheimer’s disease and down syndrome, Electrophoresis 22:172–179
Yu T-Y, Raschle T, Hiller S, Wagner G (2012) Solution NMR spectroscopic characterization of human VDAC-2 in detergent micelles and lipid bilayer nanodiscs. BBA-Biomembranes 1818:1562–1569
Zachariae U, Schneider R, Briones R, Gattin Z, Demers J-P, Giller K, Maier E, Zweckstetter M, Griesinger C, Becker S, Benz R, de Groot BL, Lange A (2012) β-Barrel mobility underlies closure of the voltage-dependent anion channel. Structure 20:1540–1549
Zhong L, Bamm VV, Ahmed MAM, Harauz G, Ladizhansky V (2007) Solid-state NMR spectroscopy of 18.5 kDa myelin basic protein reconstituted with lipid vesicles: Spectroscopic characterisation and spectral assignments of solvent-exposed protein fragments. Biochim Biophys Acta 1768:3193–3205
Zizi M, Forte M, Blachly-Dyson E, Colombini M (1994) NADH regulates the gating of VDAC, the mitochondrial outer membrane channel. J Biol Chem 269:1614–1616
Acknowledgements
This research was supported by NIH Grants EB001960 and EB002026. MTE acknowledges support from an American Cancer Society postdoctoral research fellowship. The authors thank Mr. Frans Ricardo for MALDI mass spectrometry characterization of VDAC2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eddy, M.T., Yu, TY., Wagner, G. et al. Structural characterization of the human membrane protein VDAC2 in lipid bilayers by MAS NMR. J Biomol NMR 73, 451–460 (2019). https://doi.org/10.1007/s10858-019-00242-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-019-00242-8