Abstract
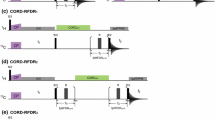
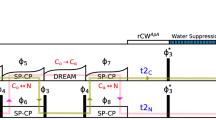
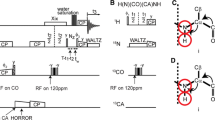
We discuss the optimum experimental conditions to obtain assignment spectra for solid proteins at magic-angle spinning (MAS) frequencies around 100 kHz. We present a systematic examination of the MAS dependence of the amide proton T 2′ times and a site-specific comparison of T 2′ at 93 kHz versus 60 kHz MAS frequency. A quantitative analysis of transfer efficiencies of building blocks, as they are used for typical 3D experiments, was performed. To do this, we compared dipolar-coupling and J-coupling based transfer steps. The building blocks were then combined into 3D experiments for sequential resonance assignment, where we evaluated signal-to-noise ratio and information content of the different 3D spectra in order to identify the best assignment strategy. Based on this comparison, six experiments were selected to optimally assign the model protein ubiquitin, solely using spectra acquired at 93 kHz MAS. Within 3 days of instrument time, the required spectra were recorded from which the backbone resonances have been assigned to over 96 %.






















Similar content being viewed by others
References
Agarwal V et al (2013) Amplitude-modulated low-power decoupling sequences for fast magic-angle spinning NMR. Chem Phys Lett 583:1–7
Agarwal V et al (2014) De Novo 3D structure determination from sub-milligram protein samples by solid-state 100 kHz MAS NMR spectroscopy. Angew Chem Int Ed 53(45):12253–12256
Andrew ER, Bradbury A, Eades RG (1958) Nuclear magnetic resonance spectra from a crystal rotated at high speed. Nature 182(4650):1659
Baldus M, Meier BH (1996) Total correlation spectroscopy in the solid state. The use of scalar couplings to determine the through-bond connectivity. J Magn Reson Ser A 121(1):65–69
Barbet-Massin E et al (2013) Out-and-back 13C-13C scalar transfers in protein resonance assignment by proton-detected solid-state NMR under ultra-fast MAS. J Biomol NMR 56(4):379–386
Barbet-Massin E et al (2014) Rapid proton-detected NMR assignment for proteins with fast magic angle spinning. J Am Chem Soc 136(35):12489–12497
Bayro MJ et al (2009) Dipolar truncation in magic-angle spinning NMR recoupling experiments. J Chem Phys 130(11):114506
Böckmann A et al (2009) Characterization of different water pools in solid-state NMR protein samples. J Biomol NMR 45(3):319–327
Bodenhausen G, Ruben DJ (1980) Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. Chem Phys Lett 69(1):185–189
Castellani F et al (2002) Structure of a protein determined by solid-state magic-angle-spinning NMR spectroscopy. Nature 420(6911):98–102
Cavanagh J et al (2007) Protein NMR spectroscopy—principle and practice. In: Cavanagh J et al (eds) Protein NMR spectroscopy, 2nd edn. Academic Press, Burlington, pp 781–817
Chen L et al (2007a) Backbone assignments in solid-state proteins using J-based 3D heteronuclear correlation spectroscopy. J Am Chem Soc 129(35):10650–10651
Chen L et al (2007b) J-based 2D homonuclear and heteronuclear correlation in solid-state proteins. Magn Reson Chem 45(Suppl 1):S84–S92
Emsley L, Bodenhausen G (1990) Gaussian pulse cascades: new analytical functions for rectangular selective inversion and in-phase excitation in NMR. Chem Phys Lett 165(6):469–476
Grzesiek S, Bax A (1992) Improved 3D triple-resonance NMR techniques applied to a 31 kDa protein. J Magn Reson (1969) 96(2):432–440
Hartmann SR, Hahn EL (1962) Nuclear double resonance in the rotating frame. Phys Rev 128(5):2042–2053
Hediger S, Meier BH, Ernst RR (1995) Adiabatic passage Hartmann-Hahn cross polarization in NMR under magic angle sample spinning. Chem Phys Lett 240(5–6):449–456
Huber M et al (2014) Solid-state NMR sequential assignment of Osaka-mutant amyloid-beta (Aβ1–40 E22Δ) fibrils. Biomol NMR Assign 9:7–14
Igumenova TI et al (2004) Assignments of carbon NMR resonances for microcrystalline ubiquitin. J Am Chem Soc 126(21):6720–6727
Kay LE et al (1990) Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J Magn Reson (1969) 89(3):496–514
Knight MJ et al (2011) Fast resonance assignment and fold determination of human superoxide dismutase by high-resolution proton-detected solid-state MAS NMR spectroscopy. Angew Chem Int Ed Engl 50(49):11697–11701
Lesage A, Bardet M, Emsley L (1999) Through-bond carbon–carbon connectivities in disordered solids by NMR. J Am Chem Soc 121(47):10987–10993
Lewandowski JZR et al (2011a) Enhanced resolution and coherence lifetimes in the solid-state NMR spectroscopy of perdeuterated proteins under ultrafast magic-angle spinning. J Phys Chem Lett 2(17):2205–2211
Lewandowski JR et al (2011b) Site-specific measurement of slow motions in proteins. J Am Chem Soc 133(42):16762–16765
Lienin SF et al (1998) Anisotropic intramolecular backbone dynamics of ubiquitin characterized by NMR relaxation and MD computer simulation. J Am Chem Soc 120(38):9870–9879
Linser R, Fink U, Reif B (2008) Proton-detected scalar coupling based assignment strategies in MAS solid-state NMR spectroscopy applied to perdeuterated proteins. J Magn Reson 193(1):89–93
Lowe IJ, Norberg RE (1957) Free-induction decays in solids. Phys Rev 107(1):46–61
Luckgei N et al (2014) Solid-state NMR sequential assignments of the C-terminal oligomerization domain of human C4b-binding protein. Biomol NMR Assign 8(1):1–6
Pauli J et al (2001) Backbone and side-chain 13C and 15N signal assignments of the α-Spectrin SH3 domain by magic angle spinning solid-state NMR at 17.6 Tesla. ChemBioChem 2(4):272–281
Pines A, Gibby MG, Waugh JS (1973) Proton-enhanced NMR of dilute spins in solids. J Chem Phys 59(2):569–590
Sattler M, Schleucher J, Griesinger C (1999) Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog Nucl Magn Reson Spectrosc 34(2):93–158
Schanda P, Meier BH, Ernst M (2010) Quantitative analysis of protein backbone dynamics in microcrystalline ubiquitin by solid-state NMR spectroscopy. J Am Chem Soc 132(45):15957–15967
Scholz I, Meier BH, Ernst M (2007) Operator-based triple-mode Floquet theory in solid-state NMR. J Chem Phys 127(20):204504
Schuetz A et al (2010) Protocols for the sequential solid-state NMR spectroscopic assignment of a uniformly labeled 25 kDa protein: HET-s(1-227). ChemBioChem 11(11):1543–1551
Shaka AJ et al (1983) An improved sequence for broadband decoupling: WALTZ-16. J Magn Reson (1969) 52(2):335–338
Stevens T et al (2011) A software framework for analysing solid-state MAS NMR data. J Biomol NMR 51(4):437–447
Thakur RS, Kurur ND, Madhu PK (2006) Swept-frequency two-pulse phase modulation for heteronuclear dipolar decoupling in solid-state NMR. Chem Phys Lett 426(4–6):459–463
Verel R et al (1998) A homonuclear spin-pair filter for solid-state NMR based on adiabatic-passage techniques. Chem Phys Lett 287(3–4):421–428
Verel R, Ernst M, Meier BH (2001) Adiabatic dipolar recoupling in solid-state NMR: the DREAM scheme. J Magn Reson 150(1):81–99
Vijay-Kumar S, Bugg CE, Cook WJ (1987) Structure of ubiquitin refined at 1.8 Å resolution. J Mol Biol 194(3):531–544
Westfeld T et al (2012) Properties of the DREAM scheme and its optimization for application to proteins. J Biomol NMR 53(2):103–112
Wittekind M, Mueller L (1993) HNCACB, a high-sensitivity 3D NMR experiment to correlate amide-proton and nitrogen resonances with the alpha- and beta-carbon resonances in proteins. J Magn Reson Ser B 101(2):201–205
Yamazaki T et al (1994) A Suite of triple resonance NMR experiments for the backbone assignment of 15 N, 13C, 2H labeled proteins with high sensitivity. J Am Chem Soc 116(26):11655–11666
Ye YQ et al (2014) Rapid measurement of multidimensional 1H solid-state NMR spectra at ultra-fast MAS frequencies. J Magn Reson 239:75–80
Zhou DH, Rienstra CM (2008) High-performance solvent suppression for proton detected solid-state NMR. J Magn Reson 192(1):167–172
Zhou Z et al (2007) A new decoupling method for accurate quantification of polyethylene copolymer composition and triad sequence distribution with 13C NMR. J Magn Reson 187(2):225–233
Acknowledgments
We thank Riccardo Cadalbert for expressing the protein and Emilie Testori and Alons Lends for help with crystallization and rotor filling. This work was supported by the Agence Nationale de la Recherche (ANR-11-BSV8-021-01, ANR-12-BS08-0013-01), the ETH Zurich, the Swiss National Science Foundation (Grant 200020_124611), the Centre National de la Recherche Scientifique (CNRS), PUT126 and ETF9229 from Estonian Ministry of Science and Education.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Penzel, S., Smith, A.A., Agarwal, V. et al. Protein resonance assignment at MAS frequencies approaching 100 kHz: a quantitative comparison of J-coupling and dipolar-coupling-based transfer methods. J Biomol NMR 63, 165–186 (2015). https://doi.org/10.1007/s10858-015-9975-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-015-9975-y