Abstract
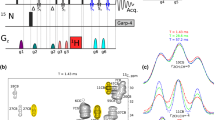
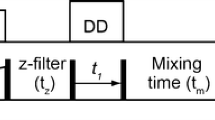
Simple pulse schemes are presented for the measurement of methyl 13C and 1H CSA values from 1H–13C dipole/13C CSA and 1H–13C dipole/1H CSA cross-correlated relaxation. The methodology is applied to protein L and malate synthase G. Average 13C CSA values are considerably smaller for Ile than Leu/Val (17 vs 25 ppm) and are in good agreement with previous solid state NMR studies of powders of amino acids and dipeptides and in reasonable agreement with quantum-chemical DFT calculations of methyl carbon CSA values in peptide fragments. Small averaged 1H CSA values on the order of 1 ppm are measured, consistent with a solid state NMR determination of the methyl group 1H CSA in dimethylmalonic acid.
Similar content being viewed by others
References
Ahlrichs, R. and Arnim, M. (1995) In Methods and Techniques in Computational Chemistry: METECC-95, Clementi, E. and Corongiu, G. (Eds.).
R. Ahlrichs M. Bär M. Häser H. Horn C. Kölmel (1989) Chem. Phys. Lett. 162 165
G. Cornilescu J. Marquardt M. Ottiger A. Bax (1998) J. Am. Chem. Soc 120 6836–6837
F. Delaglio S. Grzesiek G. W. Vuister G. Zhu J. Pfeifer A. Bax (1995) J. Biomol. NMR 6 277–293
T.M. Duncan (1990) A Compilation of Chemical Shift Anisotropies The Farragut Press Chicago
K.H. Gardner L.E. Kay (1997) J. Am. Chem. Soc. 119 7599–7600
K.H. Gardner M.K. Rosen L.E. Kay (1997) Biochemistry 36 1389–1401
K.H. Gardner X. Zhang K. Gehring L.E. Kay (1998) J. Am. Chem. Soc. 120 11738–11748
N. Godbout D.R. Salahub J. Andzelm E. Wimmer (1992) Can J. Chem. 70 560–571
N.K. Goto K.H. Gardner G.A. Mueller R.C. Willis L.E. Kay (1999) J Biomol. NMR 13 369–374
B.R. Howard J.A. Endrizzi S.J. Remington (2000) Biochemistry 39 3156–3168
R. Ishima A.P. Petkova J.M. Louis D.A. Torchia (2001) J. Am. Chem. Soc. 123 6164–6171
L.E. Kay T.E. Bull (1992) J. Magn. Reson. 99 615–622
Kay, L.E. and Prestegard, J.H. (1987) J. Am. Chem. Soc., 3829–3835.
L.E. Kay D.A. Torchia (1991) J. Magn. Reson. 95 536–547
L.E. Kay T.E. Bull L.K. Nicholson C. Griesinger H. Schwalbe A. Bax D.A. Torchia (1992) J. Magn. Reson. 100 538–558
D.M. Korzhnev K. Kloiber V. Kanelis V. Tugarinov L.E. Kay (2004) J. Am. Chem. Soc. 126 3964–3973
C.D. Kroenke J.P. Loria L.K. Lee M. Rance A.G. Palmer (1998) J. Am. Chem. Soc. 120 7905–7915
Kutzelnigg, W., Fleischer, U. and Schindler, M. (1991) In NMR: Basic Principles and Progress, Diehl, P., Fluck, E. and Kosfeld, E. (Eds.), Springer, Berlin, pp. 165–262.
M. Levitt R. Freeman (1978) J. Magn. Reson. 33 473–476
W. Liu Y. Zheng D.P. Cistola D. Yang (2003) J. Biomol. NMR 27 351–364
V.G. Malkin O.L. Malkina M.E. Casida D.R. Salahub (1994) J. Am. Chem. Soc. 116 5898–5908
Malkin, V.G., Malkina, O.L., Eriksson, L.A. and Salahub, D.R. (1995a) In Modern Density Functional Theory: A Tool for Chemists, Seminario, J.M. and Politzer, P. (Eds.), Elsevier, Amsterdam.
Malkin, V.G., Malkina, O.L. and Salahub, D.R. (1995b) In Theoretical and Computational Chemistry, Seminario, J.M. and Politzer, P. (Eds.), Elsevier, Amsterdam.
V.G. Malkin O.L. Malkina D.R. Salahub (1996) Chem. Phys. Lett. 261 335–345
D. Marion M. Ikura R. Tschudin A. Bax (1989) J. Magn. Reson. 85 393
W.J. Metzler M. Wittekind V. Goldfarb L. Mueller B.T. Farmer (1996) J. Am. Chem. Soc. 118 6800–6801
O. Millet D.R. Muhandiram N.R. Skrynnikov L.E. Kay (2002) J. Am. Chem. Soc. 124 6439–6448
A. Mittermaier L.E. Kay (1999) J. Am. Chem. Soc. 121 10608–10613
A. Mittermaier L.E. Kay (2002) J. Biomol. NMR 23 35–45
G.A. Mueller W.Y. Choy D. Yang J.D. Forman-Kay R.A. Venters L.E. Kay (2000) J. Mol. Biol. 300 197–212
D.R. Muhandiram T. Yamazaki B.D. Sykes L.E. Kay (1995) J. Am. Chem. Soc. 117 11536–11544
N. Muller G. Bodenhausen R.R. Ernst (1987) J. Magn. Reson. 75 297–334
J.E. Ollerenshaw V. Tugarinov L.E. Kay (2003) Magn. Reson. Chem. 41 843–852
M. Ottiger A. Bax (1999) J. Am. Chem. Soc. 121 4690–4695
A.G. Palmer P.E. Wright M. Rance (1991) Chem. Phys. Lett. 185 41–46
J.P. Perdew Y. Wang (1992) Phys. Rev. B 45 13244–13249
R. Richarz K. Nagayama K. Wüthrich (1980) Biochemistry 19 5189–5196
M.K. Rosen K.H. Gardner R.C. Willis W.E. Parris T. Pawson L.E. Kay (1996) J. Mol. Biol. 263 627–636
Salahub, D.R., Fournier, R., Mlynarski, P., Papai, I., St-Amant, A. and Ushio, J. (1991) In Density Functional Methods in Chemistry, Labanowski, A. and Andzelm, J. (Eds.), Springer, New York, p. 77.
J. Santoro G.C. King (1992) J. Magn. Reson. 97 202–207
M.L. Scalley Q. Yi H. Gu A. McCormack J.R. Yates D. Baker (1997) Biochemistry 36 3373–3382
W. Scheubel H. Zimmerman U. Haeberlen (1988) J. Magn. Reson. 80 401–416
C. Scheurer N.R. Skrynnikov S.F. Lienin S.K. Straus R. Brüschweiler R.R. Ernst (1999) J. Am. Chem. Soc. 121 4242–4251
A.J. Shaka J. Keeler T. Frenkiel R. Freeman (1983) J. Magn. Reson. 52 335–338
N.R. Skrynnikov O. Millet L.E. Kay (2002) J. Am. Chem. Soc. 124 6449–6460
C. Sosa J. Andzelm B.C. Elkin E. Wimmer K.D. Dobbs D.A. Dixon (1992) J. Phys. Chem. 96 6630–6636
N. Tjandra A. Bax (1997a) J. Am. Chem. Soc. 119 9576–9577
N. Tjandra A. Bax (1997b) J. Am. Chem. Soc. 119 8076–8082
V. Tugarinov L.E. Kay (2003a) J. Am. Chem. Soc. 125 13868–13878
V. Tugarinov L.E. Kay (2003b) J. Biomol. NMR 28 165–172
V. Tugarinov L.E. Kay (2004) J. Biomol. NMR 29 369–376
V. Tugarinov P.M. Hwang J.E. Ollerenshaw L.E. Kay (2003) J. Am. Chem. Soc. 125 10420–10428
V. Tugarinov R. Muhandiram A. Ayed L.E. Kay (2002) J. Am. Chem. Soc. 124 10025–10035
R.L. Vold R.R. Vold (1978) Prog. NMR Spectrosc. 12 79–133
G.W. Vuister A. Bax (1992) J. Magn. Reson. 98 428–435
L.G. Werbelow D.M. Grant (1977) Adv. Magn. Reson. 9 189–299
Werbelow, L.G. and Marshall, A.G. (1973) J. Magn. Reson., 299–313.
C. Ye R. Fu J. Hu L. Hou S. Ding (1993) Magn. Reson. Chem. 31 699–704
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tugarinov, V., Scheurer, C., Brüschweiler, R. et al. Estimates of methyl 13C and 1H CSA values (Δσ) in proteins from cross-correlated spin relaxation. J Biomol NMR 30, 397–406 (2004). https://doi.org/10.1007/s10858-004-4349-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10858-004-4349-x