Abstract
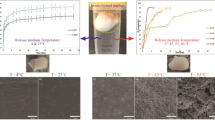
The kinetics of degradation and sustained cancer drugs (paclitaxel (PT) and prodigiosin (PG)) release are presented for minirods (each with diameter of ~5 and ~6 mm thick). Drug release and degradation mechanisms were studied from solvent-casted cancer drug-based minirods under in vitro conditions in phosphate buffer solution (PBS) at a pH of 7.4. The immersed minirods were mechanically agitated at 60 revolutions per minute (rpm) under incubation at 37 °C throughout the period of the study. The kinetics of drug release was studied using ultraviolet visible spectrometry (UV-Vis). This was used to determine the amount of drug released at 535 nm for poly(lactic-co-glycolic acid) loaded with prodigiosin (PLGA-PG) samples, and at 210 nm, for paclitaxel-loaded samples (PLGA-PT). The degradation characteristics of PLGA-PG and PLGA-PT are elucidated using optical microscope as well as scanning electron microscope (SEM). Statistical analysis of drug release and degradation mechanisms of PLGA-based minirods were performed. The implications of the results are discussed for potential applications in implantable/degradable structures for multi-pulse cancer drug delivery.




Similar content being viewed by others
References
Alwan A. Global status report on noncommunicable diseases in 2010. Geneva: World Health Organization; 2011. p. 164
Ferlay J, Shin HR, Bray F, Forman D, Mathers CD, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J of Cancer. 2010;127:2893–917.
Mathers DC, Loncar D. Updated projections of global mortality and burden of disease, 2002–2030; data sources, methods and results. Geneva, Switzerland: World Health Organization; 2005. p. 6.
Borgstede JP, Bagrosky BM. Early diagnosis and treatment of cancer series: breast cancer: screening of high-risk patients. Philadelphia: Saunders Elsevier; 2011.
Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, Conant EF, Fajardo LL, Bassett L, D’Orsi C, Jong R, Rebner M. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–83.
Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, Smith RA, Warner E, Yaffe M, Adrews KS, Russell CA. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89.
Smith RA, Saslow D, Sawyer KA, Burke W, Costanza ME, Evans WP III, Foster RS, Hendrick E, Eyre HJ, Sener S. American cancer society guidelines for breast cancer screening. CA Cancer J Clin. 2003;53:141–69.
Breast Cancer Signs & Symptoms. In: Early symptoms. What health, healthy living for everybody. 2016. http://www.whathealth.com/breastcancer/symptoms.html. Accessed 15 Dec 2016.
David N, Mark WD. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv Drug Deliv Rev. 2001;53:285–305.
Hildebrandt B, Wustin P, Ceelen WP. Peritoneal carcinomatosis: a multidisciplinary approach. New York, NY: Springer; 2007.
Oni Y, Theriault C, Hoek AV, Soboyejo WO. Effects of temperature on diffusion from PNIPA-based gels in a BioMEMS device for localized chemotherapy and hyperthermia. Mater Sci Eng C. 2011;31:67–76.
Oni Y, Soboyejo WO. Swelling and diffusion of pnipa-based gels for localized chemotherapy and hyperthermia. Mater Sci Eng C. 2012;32:24–30.
Kavanagh BD, Timmerman RD. Stereotactic body radiation therapy. 1st ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
Del Regato JA. Radiological oncologists: the unfolding of a medical specialty. Reston (VA): Radiology Centennial Inc., University of Michigan, USA; 1993;268: 167–76.
Xu M, Qian J, Liu X, Liu T, Wang H. Stimuli-responsive pegylated prodrugs for targeted doxorubicin delivery. Mater Sci Eng C. 2015;50:341–47.
Danyuo Y, Ani CJ, Obayemi JD, Dozie-Nwachukwu S, Odusanya OS, Oni Y, Anuku N, Malatesta K, Soboyejo WO. Advanced Materials for Sustainable Development. In: Soboyejo W, Odusanya S, Kana Z, Anukwu N, Malatesta K, Dauda M, editors. Prodigiosin release from an implantable biomedical device: effect on cell viability. Switzerland: Trans Tech Publications; 2016. p. 3–18.
Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99:3181–98.
Danyuo Y, Obayemi JD, Dozie-Nwachukwu S, Ani CJ, Odusanya OS, Oni Y, Anuku N, Malatesta K, Soboyejo WO. Prodigiosin release from an implantable biomedical device: kinetics of localized cancer drug release. J Mater Sci Eng C. 2014;42(1):734–45.
Makadia HK, Siege SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3(3):1377–97.
Allison SD. Effect of structural relaxation on the preparation and drug release behavior of poly(lactic-co-glycolic)acid microparticle drug delivery systems. J Pharm Sci. 2008;97:2022–35.
Mundargi R, Babu V, Rangaswamy V, Patel P, Aminabhavi T. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J Cont Rel. 2008;125:193–209.
Mohamed F, van der Walle CF. Engineering biodegradable polyester particles with specific drug targeting and drug release properties. J Pharm Sci. 2008;97:71–87.
Metters AT, Bowman CN. A statistical kinetic model for the bulk degradation of PLA-b-PEG-b-PLA hydrogel networks; incorporating network non-idealities. J Phys Chem B. 2001;104:8069–76.
Obayemi JD, Danyuo Y, Dozie-Nwachukwu S, Odusanya OS, Anuku N, Malatesta K, Yu W, Uhrich KE, Soboyejo WO. PLGA-based microparticles loaded with bacterial-synthesized prodigiosin for anticancer drug release: effects of particle size on drug release kinetics and cell viability. Mater Sci Eng C. 2016;66:51–65.
Behravesh E, Yasko AW, Engel PS, Mikos AG. Synthetic biodegradable polymers for orthopaedic applications. Clin Orthop Relat Res. 1999;367:118–25.
Middleton E Jr., Kandaswami C, Theoharide TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000;52(4):673–751.
Park TG. Degradation of poly (D,L-lactic acid) microspheres: effect of molecular weight. J Control Release. 1994;30:161–73.
Metzmacher I, Radu F, Bause M, Knabner P, Friess W. A model describing the effect of enzymatic degradation on drug release from collagen minirods. Eur J Pharm Biopharm. 2007;67(2):349–60.
Danyuo Y, Dozie-Nwachukwu S, Obayemi JD, Ani CJ, Odusanya OS, Oni Y, Anuku N, Malatesta K, Soboyejo WO. Swelling of poly(N-isopropyl acrylamide) (PNIPA)-based hydrogels with bacterial-synthesized prodigiosin for localized cancer drug delivery. Mater Sci Engr C. 2016;59:19–29.
Flanagan RJ, Taylor AA, Watson ID, Whelpton R. Fundamentals of analytical toxicology. Newyork, USA: Wiley and Sons; 2007. p. 1–486.
Pathiraja AG, Raju A. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;6:1–16.
Koleske JV. Blends containing poly(∈-caprolactone) and related polymers. In: Paul R, Newman S, editors. Polymer Blends. New York, NY: Academic; 1978. p. 369–89.
Peppas AN. Analysis of fickian and non-fickian drug release from polymers. Pharm Acta Helv. 1985;60(4):110–11.
Danyuo Y, Obayemi JD, Dozie-Nwachukwu S, Ani CJ, Odusanya OS, Oni Y, Anuku N, Malatesta K, Soboyejo WO. Prodigiosin release from an implantable biomedical device: kinetics of localized cancer drug release. Mater Sci Eng C. 2014;42(1):734–45.
Ogunnaike BA. Random phenomena, fundamentals of probability & statistics for engineers. Newark: University of Delaware; 2009.
McDonald JH. Handbook of biological statistics. 3rd ed. Maryland: Sparky House Publishing; 2014.
Thomas CDL, Feik SA and Clement JG. Increase in pore area, and not pore density, is the main determinant in the development of porosity in human cortical bone. J Anat. 2006;209:219–30.
Obayemi JD, Danyuo Y, Dozie-Nwachukwu S, Odusanya OS, Anuku N, Malatesta K, Yu W, Uhrich KE, Soboyejo WO. PLGA-based microparticles loaded with bacterial-synthesized prodigiosin for anticancer drug release: effects of particle size on drug release kinetics and cell viability. Mater Sci Eng C. 2016;66:51–65.
Tamagawa H, Popovic S, Taya M. Pores and diffusion characteristics of porous gels. Polymer. 2000;41:7201–7.
Bae SE, Sona JS, Parka K, Han DK. Fabrication of covered porous PLGA microspheres using hydrogen peroxide for controlled drug delivery and regenerative medicine. J Control Release. 2009;133(1):37–43.
Chen J, Lee J-W, Hernandez de Gatica NL, Burkhardt CA, Hercules DM, Gardella JA Jr. Time-of-flight secondary ion mass spectrometry studies of hydrolytic degradation kinetics at the surface of poly(glycolic acid). Macromolecules. 2000;33:4726–32.
Klose D, Siepmann F. PLGA-based drug delivery systems: importance of the type of drug and device geometry. Int J Pharm. 2008;354:95–103.
Engineer C, Parikh J, Raval A. Review on hydrolytic degradation behavior of biodegradable polymers from controlled drug delivery system. Trends Biomater Artif Organs. 2011;25(2):79–85.
Acknowledgements
The authors are very grateful to the World Bank African Centres of Excellent Project (Pan-African Materials Institute (PAMI) with grant No. P126974), the African Capacity Building Foundation with Grant No. 292, Princeton University School of Engineering and Applied Sciences (SEAS) and the Worcester Polytechnic Institute for financial support. The authors are also grateful to the Nelson Mandela Institute (NMI) and the African University of Science and Technology (AUST) for the scholarships given to the students. Appreciation is also extended to Prof. Eric Garfunkel and Dr. Adeoye Soyemi for useful technical discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Danyuo, Y., E. Oberaifo, O., Obayemi, J.D. et al. Extended pulsated drug release from PLGA-based minirods. J Mater Sci: Mater Med 28, 61 (2017). https://doi.org/10.1007/s10856-017-5866-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-017-5866-y