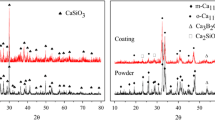
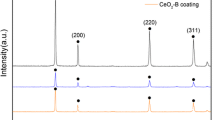
Abstract
Mg2+, Zn2+ and Sr2+ substitution for Ca2+ in plasma sprayed calcium silicate (Ca–Si) coatings have been reported to impede their degradation in physiological environment and, more importantly, to improve their biological performance. The reason for the improved biological performance is still elusive and, especially, the contribution of the dopant ions is lack of obvious and direct evidence. In this study, we aim to identify the effect of Mg2+, Zn2+ and Sr2+ incorporation on the osteogenic ability of Ca–Si based coatings (Ca2MgSi2O7, Ca2ZnSi2O7 and Sr-CaSiO3) by minimizing the influence of Ca and Si ions release and surface physical properties. Similar surface morphology, crystallinity and roughness were achieved for all samples by optimizing the spray parameters. As expected, Ca and Si ions release from all the coatings showed the comparable concentration with immersing time. The response of MC3T3-E1 cells onto Mg2+, Zn2+ and Sr2+ doped Ca–Si coatings were studied in terms of osteoblastic adhesion, proliferation, differentiation and mineralization. The results showed that the level of cell adhesion and proliferation increased the most on the surface of Mg-modified coating. Gene expressions of early markers of osteoblast differentiation (COL-I and ALP mRNA) were obviously improved on Zn-modified coating. Gene expressions of later markers for osteoblast differentiation (OPN and OC mRNA) and mineralized nodules formation were obviously accelerated on the surface of Sr-modified coating. Since Mg2+, Zn2+ and Sr2+ play a regulatory role in different stages of osteogenesis, it may be possible to utilize this in the development of new coating materials for orthopedic application.














Similar content being viewed by others
References
Ni S, Chang J, Chou L, Zhai W. Comparison of osteoblast-like cell responses to calcium silicate and tricalcium phosphate ceramics in vitro. J Biomed Mater Res B Appl Biomater. 2007;80(1):174–83. doi:10.1002/jbm.b.30582.
Xu S, Lin K, Wang Z, Chang J, Wang L, Lu J, et al. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials. 2008;29(17):2588–96. doi:10.1016/j.biomaterials.2008.03.013.
Liu X, Morra M, Carpi A, Li B. Bioactive calcium silicate ceramics and coatings. Biomed Pharmacother. 2008;62(8):526–9. doi:10.1016/j.biopha.2008.07.051.
Mohammadi H, Hafezi M, Nezafati N, Heasarki S, Nadernezhad A, Ghazanfari SMH, et al. Bioinorganics in bioactive calcium silicate ceramics for bone tissue repair: bioactivity and biological properties. J Ceram Sci Technol. 2014;5(1):1–12.
Zhang NL, Molenda JA, Mankoci S, Zhou XF, Murphy WL, Sahai N. Crystal structures of CaSiO3 polymorphs control growth and osteogenic differentiation of human mesenchymal stem cells on bioceramic surfaces. Biomater Sci. 2013;1(10):1101–10. doi:10.1039/C3bm60034c.
Wu CT, Chang J. A novel akermanite bioceramic: preparation and characteristics. J Biomater Appl. 2006;21(2):119–29. doi:10.1177/0885328206057953.
Wu CT, Chang J, Zhai WY. A novel hardystonite bioceramic: preparation and characteristics. Ceram Int. 2005;31(1):27–31. doi:10.1016/j.ceramint.2004.02.008.
Wu CT, Ramaswamy Y, Kwik D, Zreiqat H. The effect of strontium incorporation into CaSiO3 ceramics on their physical and biological properties. Biomaterials. 2007;28(21):3171–81.
Liang Y, Xie Y, Ji H, Huang L, Zheng X. Excellent stability of plasma-sprayed bioactive Ca3ZrSi2O9 ceramic coating on Ti–6Al–4V. Appl Surf Sci. 2010;256(14):4677–81. doi:10.1016/j.apsusc.2010.02.071.
Wu CT, Ramaswamy Y, Soeparto A, Zreiqat H. Incorporation of titanium into calcium silicate improved their chemical stability and biological properties. J Biomed Mater Res Part A. 2008;86A(2):402–10. doi:10.1002/jbm.a.31623.
Rude RK, Gruber HE, Wei LY, Frausto A, Mills BG. Magnesium deficiency: effect on bone and mineral metabolism in the mouse. Calcif Tissue Int. 2003;72(1):32–41.
Yamaguchi M. Role of zinc in bone formation and bone resorption. J Trace Elem Exp Med. 1998;11(2–3):119–35.
Pors Nielsen S. The biological role of strontium. Bone. 2004;35(3):583–8. doi:10.1016/j.bone.2004.04.026.
Lakhkar NJ, Lee IH, Kim HW, Salih V, Wall IB, Knowles JC. Bone formation controlled by biologically relevant inorganic ions: role and controlled delivery from phosphate-based glasses. Adv Drug Deliv Rev. 2013;65(4):405–20. doi:10.1016/j.addr.2012.05.015.
Reffitt DM, Ogston N, Jugdaohsingh R, Cheung HFJ, Evans BAJ, Thompson RPH, et al. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32(2):127–35. doi:10.1016/s8756-3282(02)00950-x.
Shie MY, Ding SJ, Chang HC. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011;7(6):2604–14. doi:10.1016/j.actbio.2011.02.023.
Zhai W, Lu H, Wu C, Chen L, Lin X, Naoki K, et al. Stimulatory effects of the ionic products from Ca-Mg-Si bioceramics on both osteogenesis and angiogenesis in vitro. Acta Biomater. 2013;9(8):8004–14. doi:10.1016/j.actbio.2013.04.024.
Xue W, Liu X, Zheng X, Ding C. Dissolution and mineralization of plasma-sprayed wollastonite coatings with different crystallinity. Surf Coat Technol. 2005;200(7):2420–7. doi:10.1016/j.surfcoat.2004.07.114.
Lazaro GS, Santos SC, Resende CX, dos Santos EA. Individual and combined effects of the elements Zn, Mg and Sr on the surface reactivity of a SiO2 center dot CaO center dot Na2O center dot P2O5 bioglass system. J Non-Cryst Solids. 2014;386:19–28. doi:10.1016/j.jnoncrysol.2013.11.038.
Kieswetter KS. Z; Dean, DD; Boyan, BD. The role of implant surface characteristics in the healing of bone. Crit Rev Oral Biol Med. 1996;7(4):329–45.
Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32(11):2757–74. doi:10.1016/j.biomaterials.2011.01.004.
Rezania A, Healy KE. Integrin subunits responsible for adhesion of human osteoblast-like cells to biomimetic peptide surfaces. J Orthopaed Res. 1999;17(4):615–23.
Zreiqat H, Howlett CR, Zannettino A, Evans P, Schulze-Tanzil G, Knabe C, et al. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J Biomed Mater Res. 2002;62(2):175–84.
Aina V, Perardi A, Bergandi L, Malavasi G, Menabue L, Morterra C, et al. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblasts. Chem Biol Interact. 2007;167(3):207–18. doi:10.1016/j.cbi.2007.03.002.
Li K, Yu J, Xie Y, Huang L, Ye X, Zheng X. Effects of Zn content on crystal structure, cytocompatibility, antibacterial activity, and chemical stability in zn-modified calcium silicate coatings. J Therm Spray Technol. 2013;22(6):965–73. doi:10.1007/s11666-013-9938-3.
Hall SL, Dimai HP, Farley JR. Effects of zinc on human skeletal alkaline phosphatase activity in vitro. Calcif Tissue Int. 1999;64(2):163–72. doi:10.1007/s002239900597.
Yamaguchi M, Goto M, Uchiyama S, Nakagawa T. Effect of zinc on gene expression in osteoblastic MC3T3-E1 cells: enhancement of Runx2, OPG, and regucalcin mRNA expressions. Mol Cell Biochem. 2008;312(1–2):157–66. doi:10.1007/s11010-008-9731-7.
Seo HJ, Cho YE, Kim T, Shin HI, Kwun IS. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr Res Pract. 2010;4(5):356–61. doi:10.4162/nrp.2010.4.5.356.
Zhang J, Ma X, Lin D, Shi H, Yuan Y, Tang W, et al. Magnesium modification of a calcium phosphate cement alters bone marrow stromal cell behavior via an integrin-mediated mechanism. Biomaterials. 2015;53:251–64. doi:10.1016/j.biomaterials.2015.02.097.
Saidak Z, Marie PJ. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther. 2012;136(2):216–26. doi:10.1016/j.pharmthera.2012.07.009.
Xue W, Moore JL, Hosick HL, Bose S, Bandyopadhyay A, Lu WW, et al. Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics. J Biomed Mater Res Part A. 2006;79(4):804–14. doi:10.1002/jbm.a.30815.
Barbara A, Delannoy P, Denis BG, Marie PJ. Normal matrix mineralization induced by strontium ranelate in MC3T3-E1 osteogenic cells. Metabolism. 2004;53(4):532–7. doi:10.1016/j.metabol.2003.10.022.
Dahl SG, Allain P, Marie PJ, Mauras Y, Boivin G, Ammann P, et al. Incorporation and distribution of strontium in bone. Bone. 2001;28(4):446–53. doi:10.1016/S8756-3282(01)00419-7.
Wu LNY, Genge BR, Wuthier RE. Differential effects of zinc and magnesium ions on mineralization activity of phosphatidylserine calcium phosphate complexes. J Inorg Biochem. 2009;103(7):948–62. doi:10.1016/j.jinorgbio.2009.04.004.
Bigi A, Foresti E, Gandolfi M, Gazzano M, Roveri N. Inhibiting effect of zinc on hydroxylapatite crystallization. J Inorg Biochem. 1995;58(1):49–58. doi:10.1016/0162-0134(94)00036-A.
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grant No. 51172264, No. 51502328) and the Opening Project of the Shanghai Key Laboratory of Orthopedic Implant (Grant No. KFKT2014002).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, D., Li, K., Xie, Y. et al. Different response of osteoblastic cells to Mg2+, Zn2+ and Sr2+ doped calcium silicate coatings. J Mater Sci: Mater Med 27, 56 (2016). https://doi.org/10.1007/s10856-016-5672-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-016-5672-y