Abstract
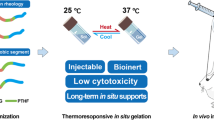
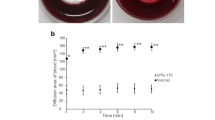
PEG-PCL-PEG (PECE) hydrogel for intracameral injection as a sustained delivery system can get a stable release of the medication and achieve an effective local concentration. The injectable PECE hydrogel is thermosensitive nano-material which is flowing sol at low temperature and can shift to nonflowing gel at body temperature. This study evaluated the intracameral injection of bevacizumab combined with a PECE hydrogel drug release system on postoperative scarring and bleb survival after experimental glaucoma filtration surgery. The best result was achieved in the bevacizumab loaded PECE hydrogels group, which presented the lowest IOP values after surgery. And the blebs were significantly more persistent in this group. Histology, Massion trichrome staining and immunohistochemistry further demonstrated that glaucoma filtration surgery in combination with bevacizumab loaded PECE hydrogel resulted in good bleb survival due to scar formation inhibition. In conclusions, this study demonstrated that bevacizumab-loaded PECE hydrogel for intracameral injection as a sustained delivery system provide a great opportunity to increase the therapeutic efficacy of glaucoma filtration surgery.




Similar content being viewed by others
References
Burr J, Azuara-Blanco A, Avenell A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2012;9:CD004399.
Hitchings R. Initial treatment for open-angle glaucoma-medical, laser, or surgical? Surgery is the treatment of choice for open-angle glaucoma. Arch Ophthalmol. 1998;116(2):241–2.
Chen CW, Huang HT, Bair JS, Lee CC. Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma. J Ocul Pharmacol. 1990;6(3):175–82.
Greenfield DS, Liebmann JM, Jee J, Ritch R. Lateonset bleb leaks after glaucoma filtering surgery. Arch Ophthalmol. 1998;116(4):443–7.
Kitazawa Y, Taniguchi T, Nakano Y, Shirato S, Yamamoto T. 5-Fluorouracil for trabeculectomy in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1987;225(6):403–5.
Paula JS, Ribeiro VR, Chahud F, Cannellini R, Monteiro TC, Gomes EC, Reinach PS, Rodrigues Mde L, Silva-Cunha A. Bevacizumab-loaded polyurethane subconjunctival implants: effects on experimental glaucoma filtration surgery. J Ocul Pharmacol Ther. 2013;29(6):566–73.
Grewal DS, Jain R, Kumar H, Grewal SPS. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy a pilot study. Ophthalmology. 2008;115(12):2141–5.
Li Z, Van Bergen T, Van de Veire S, Van de Vel I, Moreau H, Dewerchin M. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009;50(11):5217–25.
Vandewalle E, Abegão Pinto L, Van Bergen T, Spielberg L, Fieuws S, Moons L, Spileers W, Zeyen T, Stalmans I. Intracameral bevacizumab as an adjunct to trabeculectomy: a 1-year prospective, randomised study. Br J Ophthalmol. 2014;98(1):73–8.
Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K, Nugent AK, Hirooka K, Baba T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest. Ophthalmol Vis Sci. 2009;50(10):4807–13.
Gong C, Shi S, Dong P, Kan B, Gou M, Wang X, Li X, Luo F, Zhao X, Wei Y, Qian Z. Synthesis and characterization of PEG-PCL-PEG thermosensitive hydrogel. Int J Pharm. 2009;365:89–99.
Yang B, Gong C, Zhao X, Zhou S, Li Z, Qi X, Zhong Q, Luo F, Qian Z. Preventing postoperative abdominal adhesions in a rat model with PEG-PCL-PEG hydrogel. Int J Nanomed. 2012;7:547–57.
Luo Z, Jin L, Xu L, Zhang ZL, Yu J, Shi S, Li X, Chen H. Thermosensitive PEG-PCL-PEG (PECE) hydrogel as an in situ gelling system for ocular drug delivery of diclofenac sodium. Drug Deliv. 2014. doi:10.3109/10717544.2014.903535.
Yin H, Gong C, Qian Z, Liu X, Wei Y, Qian Z. Toxicity evaluation of biodegradable and thermosensitive PEG-PCL-peg hydrogel as a potential in situ sustained ophthalmic drug delivery system. J Biomed Mater Res B Appl Biomater. 2010;92(1):129–37.
Peng R, Qin G, Li X, Lv H, Qian Z, Yu L. The PEG-PCL-PEG hydrogel as an Implanted ophthalmic delivery system after glaucoma filtration surgery; a Pilot Study. Med Hypothesis Discov Innov Ophthalmol. 2014;3(1):3–8.
Kissel T, Li Y, Unger F. ABA-triblock copolymers from biodegradable polyerster A-blocks and bydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery systems for proteins. Adv Drug Deliv Rev. 2002;54:99–134.
Rodríguez-Agirretxe I, Vega SC, Rezola R, Vecino E, Mendicute J, Suarez-Cortes T, Acera A. The PLGA implant as an antimitotic delivery system after experimental trabeculectomy. Invest Ophthalmol Vis Sci. 2013;54(8):5227–35.
Okuda T, Higashide T, Fukuhira Y, Sumi Y, Shimomura M, Sugiyama K. A thin honeycomb-patterned film as an adhesion barrier in an animal model of glaucoma filtration surgery. J Glaucoma. 2009;18:220–6.
Einmahl S, Behar-Cohen F, D’Hermies F, Rudaz S, Tabatabay C, Renard G. GurnyR. A new poly(ortho ester)-based drug delivery system as an adjunct treatment in filtering surgery. Invest Ophthalmol Vis Sci. 2001;42(3):695–700.
Cagiannos C, Abul-Khoudoud OR, DeRijk W, Shell DH 4th, Jennings LK, Tolley EA, Handorf CR, Fabian TC. Rapamycin-coated expanded polytetrafluoroethylene bypass grafts exhibit decreased anastomotic neointimal hyperplasia in a porcine model. J Vasc Surg. 2005;42:980–8.
Asahara T, Bauters C, Zheng LP, Takeshita S, Bunting S, Ferrara N, Symes JF, Isner JM. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circluation. 1995;92(9 suppl):II365-71.
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–80.
Pollina EA, Legesse-Miller A, Haley EM, Goodpaster T, Randolph-Habecker J, Coller HA. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7(13):2056–70.
Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. Regulation of scar formation by vascular endothelial growth factor. Lab Invest. 2008;88(6):579–90.
Park HYL, Kim JH, Park CK. VEGF induces TGF-β1 expression and myofibroblast transformation after glaucoma surgery. Am J Pathol. 2013;182(6):2147–54.
Zhou M, Wan W, Huang W, Cheng B, Ding X, Zhang X. Levels of erythropoietin and vascular endothelial growth factor in surgery-required advanced neovascular glaucoma eyes before and after intravitreal injection of bevacizumab. Invest Ophthalmol Vis Sci. 2013;54(6):3874–9.
Kahook MY, Schuman JS, Noecker RJ. Intravitreal bevacizumab in a patient with neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37(2):144–6.
Rangelov S, Dimitrov P, Tsvetanov CB. Mixed block copolymer aggregates with tunable temperature behavior. J Phys Chem B. 2005;109(3):1162–7.
Kissel T, Li Y, Unger F. ABA-triblock copolymers from biodegradable polyerster A-blocks and bydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery systems for proteins. Adv Drug Deliv Rev. 2002;54(1):99–134.
Hwang MJ, Suh JM, Bae YH, Kim SW, Jeong B. Caprolactonic poloxamer analog: PEG-PCL-PEG. Biomacromolecules. 2005;6(2):885–90.
Lei N, Gong C, Qian Z, Luo F, Wang C, Wang H, Wei Y. Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model. Nanoscale. 2012;4(18):5686–93.
Ni P, Ding Q, Fan M, Liao J, Qian Z, Luo J, Li X, Luo F, Yang Z, Wei Y. Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials. 2014;35(1):236–48.
Gong CY, Liu CB, Qian ZY, Liu CB, Huang MJ, Gu YC, Wen YJ, Kan B, Wang K, Dai M, Li XY, Gou ML, Tu MJ, Wei YC. A thermosensitive hydrogel based on biodegradable amphiphilic poly(ethylene glycol)–polycaprolactone–poly(ethylene glycol) block copolymers. Smart Mater Struct. 2007;16:927–33.
Liu W, Griffith M, Li F. Alginate microsphere-collagen composite hydrogel for ocular drug delivery and implantation. J Mater Sci Mater Med. 2008;19(11):3365–71.
Palma SD, Tartara LI, Quinteros D, Allemandi DA, Longhi MR, Granero GE. An efficient ternary complex of acetazolamide with HP-ss-CD and TEA for topical ocular administration. J Control Release. 2009;138(1):24–31.
Karlgard CC, Wong NS, Jones LW, Moresoli C. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and pHEMA hydrogel contact lens materials. Int J Pharm. 2003;257(1–2):141–51.
Nagarwal RC, Kumar R, Dhanawat M, Pandit JK. Modified PLA nano in situ gel: a potential ophthalmic drug delivery system. Colloids Surf B Biointerfaces. 2011;86(1):28–34.
Marrink SJ, Mark AE. The mechanism of vesicle fusion as revealed by molecular dynamics simulations. J Am Chem Soc. 2003;125(37):11144–5.
Haluska CK, Riske KA, Marchi-Artzner V, Lehn JM, Lipowsky R, Dimova R. Time scales of membrane fusion revealed by direct imaging of vesicle fusion with high temporal resolution. Proc Natl Acad Sci U S A. 2006;103(43):15841–6.
Kitnarong N, Chindasub P, Metheetrairut A. Surgical outcome of intravitreal bevacizumab and filtration surgery in neovascular glaucoma. Adv Ther. 2008;25(5):438–43.
Baldassare RD, Brunette I, Desjardins DC, Amyot M. Corneal ectasia secondary to excessive ocular massage following trabeculectomy with 5-fluorouracil. Can J Ophthalmol. 1996;31:252–4.
How A, Chua JL, Charlton A, Su R, Lim M, Kumar RS, Crowston JG, Wong TT. Combined treatment with bevacizumab and 5-fluorouracil attenuates the postoperative scarring response after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2010;51(2):928–32.
Abrishami M, Zarei-Ghanavati S, Soroush D, Rouhbakhsh M, Jaafari MR, Malaekeh-Nikouei B. Preparation, characterization, and in vivo evaluation of nanoliposomes-encapsulated bevacizumab (avastin) for intravitreal administration. Retina. 2009;29:699–703.
Andrew JS, Anglin EJ, Wu EC, Chen MY, Cheng L, Freeman WR, Sailor MJ. Sustained release of a monoclonal antibody from electrochemically prepared mesoporous silicon oxide. Adv Funct Mater. 2010;20:4168–74.
Van Bergen T, Vandewalle E, Van de Veire S, Dewerchin M, Stassen JM, Moons L, Stalmans I. The role of different VEGF isoforms in scar formation after glaucoma filtration surgery. Exp Eye Res. 2011;93:689–99.
Memarzadeh F, Varma R, Lin LT, Parikh JG, Dustin L, Alcaraz A, Eliott D. Postoperative use of bevacizumab as an antifibrotic agent in glaucoma filtration surgery in the rabbit. Invest Ophthalmol Vis Sci. 2009;50(7):3233–7.
Huang W, Chen S, Gao X, Yang M, Zhang J, Li X, Wang W, Zhou M, Zhang X, Zhang X. Inflammation-related cytokines of aqueous humor in acute primary angle-closure eyes. Invest Ophthalmol Vis Sci. 2014;55(2):1088–94.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (No. 30901658) and International Science & Technology Cooperation Program of China (No. 2013DFG52300). Dr. Ling Yu and Dr. Zhiyong Qian contributed equally and are regarded as Co-Correspondence authors.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Han, Q., Wang, Y., Li, X. et al. Effects of bevacizumab loaded PEG-PCL-PEG hydrogel intracameral application on intraocular pressure after glaucoma filtration surgery. J Mater Sci: Mater Med 26, 225 (2015). https://doi.org/10.1007/s10856-015-5556-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10856-015-5556-6