Abstract
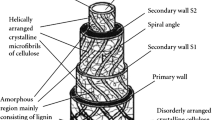
The oxidative thermal stability of plant-based microcrystalline flax fiber was developed by incorporating diammonium phosphate (DAP), boric acid, and urea (in brief DAP-BAU) followed by a multistep thermal oxidation process. By utilizing a set of measurements, including X-ray diffraction, differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), and infrared (IR) spectroscopy analysis, the impact of DAP-BAU inclusion on the thermal stability of flax fibers was studied. The findings of IR spectra and X-ray diffraction analysis revealed that the dehydrogenation and dehydration processes cause a progressive and steady loss of inter- and intramolecular H-bondings. Infrared spectra also showed the development of C = C double bonds associated with the crosslinked ladder structure formation. DSC and TGA findings revealed that DAP-BAU incorporation boosted thermal stability by generating ladder-like structure formation and restricted the development of volatile by-products by inhibiting the fundamental hydroxyl groups with increasing oxidation time. The overall findings of this study confirm that DAP-BAU incorporated and 125 min stabilized (at 245 °C) flax fibers attain complete thermal stability and are ready for utilizing in the subsequent carbonization and activation stages in activated carbon fiber manufacturing.




















Similar content being viewed by others
References
Zhang S, Shao T, Kose HS, Karanfil T (2010) Adsorption of aromatic compounds by carbonaceous adsorbents: a comparative study on granular activated carbon, activated carbon fiber, and carbon nanotubes. Environ Sci Technol 44:6377–6383. https://doi.org/10.1021/es100874y
Subramanian V, Luo C, Stephan AM et al (2007) Supercapacitors from activated carbon derived from banana fibers. J Phys Chem C 111:7527–7531. https://doi.org/10.1021/jp067009t
Nandi M, Okada K, Dutta A et al (2012) Unprecedented CO 2 uptake over highly porous N-doped activated carbon monoliths prepared by physical activation. Chem Commun 48:10283–10285. https://doi.org/10.1039/C2CC35334B
Pelekani C, Snoeyink VL (1999) Competitive adsorption in natural water: role of activated carbon pore size. Water Res 33:1209–1219. https://doi.org/10.1016/S0043-1354(98)00329-7
Alcañiz-Monge J, Linares-Solano A, Rand B (2002) Mechanism of adsorption of water in carbon micropores as revealed by a study of activated carbon fibers. J Phys Chem B 106:3209–3216. https://doi.org/10.1021/jp014388b
Cabiac A, Cacciaguerra T, Trens P et al (2008) Influence of textural properties of activated carbons on Pd/carbon catalysts synthesis for cinnamaldehyde hydrogenation. Appl Catal A Gen 340:229–235. https://doi.org/10.1016/j.apcata.2008.02.018
Caqueret V, Bostyn S, Cagnon B, Fauduet H (2008) Purification of sugar beet vinasse–adsorption of polyphenolic and dark colored compounds on different commercial activated carbons. Bioresour Technol 99:5814–5821. https://doi.org/10.1016/j.biortech.2007.10.009
Horikawa T, Hayashi J, Muroyama K (2002) Preparation of molecular sieving carbon from waste resin by chemical vapor deposition. Carbon N Y 40:709–714. https://doi.org/10.1016/S0008-6223(01)00157-9
Khomenko V, Raymundo-Piñero E, Béguin F (2008) High-energy density graphite/AC capacitor in organic electrolyte. J Power Sources 177:643–651. https://doi.org/10.1016/j.jpowsour.2007.11.101
Rosas JM, Bedia J, Rodríguez-Mirasol J, Cordero T (2009) HEMP-derived activated carbon fibers by chemical activation with phosphoric acid. Fuel 88:19–26. https://doi.org/10.1016/j.fuel.2008.08.004
Williams PT, Reed AR (2006) Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenerg 30:144–152. https://doi.org/10.1016/j.biombioe.2005.11.006
Fu R, Liu L, Huang W, Sun P (2003) Studies on the structure of activated carbon fibers activated by phosphoric acid. J Appl Polym Sci 87:2253–2261. https://doi.org/10.1002/app.11607
Zeng F, Pan D, Pan N (2005) Choosing the impregnants by thermogravimetric analysis for preparing rayon-based carbon fibers. J Inorg Organomet Polym Mater 15:261–267. https://doi.org/10.1007/s10904-005-5543-3
Zhang Z, Li J, Sun F et al (2011) Preparation and characterization of activated carbon fiber from paper. Chinese J Chem Phys 24:103–108. https://doi.org/10.1088/1674-0068/24/01/103-108
Uraki Y, Nakatani A, Kubo S, Sano Y (2001) Preparation of activated carbon fibers with large specific surface area from softwood acetic acid lignin. J Wood Sci 47:465–469. https://doi.org/10.1007/BF00767899
Asakura R, Morita M, Maruyama K et al (2004) Preparation of fibrous activated carbons from wood fiber. J Mater Sci 39:201–206. https://doi.org/10.1023/B:JMSC.0000007745.62879.74
Phan NH, Rio S, Faur C et al (2006) Production of fibrous activated carbons from natural cellulose (jute, coconut) fibers for water treatment applications. Carbon N Y 44:2569–2577. https://doi.org/10.1016/j.carbon.2006.05.048
Li K, Li Y, Hu H (2011) Adsorption characteristics of lead on cotton-stalk-derived activated carbon fibre by steam activation. Desalin Water Treat 30:1–9. https://doi.org/10.5004/dwt.2011.1130
Jimenez V, Sánchez P, Romero A (2017) Materials for activated carbon fiber synthesis. Activated carbon fiber and textiles. Elsevier, Amsterdam, pp 21–38
He D, Wu L, Yao Y et al (2020) A facile route to high nitrogen-containing porous carbon fiber sheets from biomass-flax for high-performance flexible supercapacitors. Appl Surf Sci 507:145108. https://doi.org/10.1016/j.apsusc.2019.145108
Aliotta L, Gigante V, Coltelli M-B et al (2019) Thermo-mechanical properties of PLA/short flax fiber biocomposites. Appl Sci 9:3797. https://doi.org/10.3390/app9183797
Dumanlı AG, Windle AH (2012) Carbon fibres from cellulosic precursors: a review. J Mater Sci 47:4236–4250. https://doi.org/10.1007/s10853-011-6081-8
Il KM, Park M-S, Lee Y-S (2016) Cellulose-based carbon fibers prepared using electron-beam stabilization. Carbon Lett 18:56–61. https://doi.org/10.5714/CL.2016.18.056
Fukuzumi H, Saito T, Okita Y, Isogai A (2010) Thermal stabilization of TEMPO-oxidized cellulose. Polym Degrad Stab 95:1502–1508. https://doi.org/10.1016/j.polymdegradstab.2010.06.015
Bengtsson A, Bengtsson J, Sedin M, Sjöholm E (2019) Carbon fibers from lignin-cellulose precursors: effect of stabilization conditions. ACS Sustain Chem Eng 7:8440–8448. https://doi.org/10.1021/acssuschemeng.9b00108
Byrne N, De Silva R, Ma Y et al (2018) Enhanced stabilization of cellulose-lignin hybrid filaments for carbon fiber production. Cellulose 25:723–733. https://doi.org/10.1007/s10570-017-1579-0
Gregorová A, Košíková B, Moravčík R (2006) Stabilization effect of lignin in natural rubber. Polym Degrad Stab 91:229–233. https://doi.org/10.1016/j.polymdegradstab.2005.05.009
Yue Z, Vakili A, Hosseinaei O, Harper DP (2017) Lignin-based carbon fibers: Accelerated stabilization of lignin fibers in the presence of hydrogen chloride. J Appl Polym Sci 134:45507. https://doi.org/10.1002/app.45507
Kadla JF, Kubo S, Gilbert RD, Venditti RA (2002) Lignin-Based Carbon Fibers BT - Chemical Modification, Properties, and Usage of Lignin. In: Hu TQ (ed). Springer US, Boston, MA, pp 121–137
Akpan EI (2019) Stabilization of Lignin Fibers BT - Sustainable Lignin for Carbon Fibers: Principles, Techniques, and Applications. In: Akpan EI, Adeosun SO (eds). Springer International Publishing, Cham, pp 325–352
Nam S, Condon BD, Parikh DV et al (2011) Effect of urea additive on the thermal decomposition of greige cotton nonwoven fabric treated with diammonium phosphate. Polym Degrad Stab 96:2010–2018. https://doi.org/10.1016/j.polymdegradstab.2011.08.014
Nuessle AC, Ford FM, Hall WP, Lippert AL (1956) Some aspects of the cellulose-phosphate-urea reaction. Text Res J 26:32–39. https://doi.org/10.1177/004051755602600105
Blanchard EJ, Graves EE (2003) Phosphorylation of cellulose with some phosphonic acid derivatives. Text Res J 73:22–26. https://doi.org/10.1177/004051750307300104
Chau CN, Smith JA (1992) Method of making luminescent grade boron phosphate. US Patent 5082640
Gaan S, Sun G (2007) Effect of phosphorus and nitrogen on flame retardant cellulose: a study of phosphorus compounds. J Anal Appl Pyrolysis 78:371–377. https://doi.org/10.1016/j.jaap.2006.09.010
Karacan I, Baysal G (2012) Investigation of the effect of cupric chloride on thermal stabilization of polyamide 6 as carbon fiber precursor. Fibers Polym 13:864–873. https://doi.org/10.1007/s12221-012-0864-7
Karacan I, Soy T (2013) Enhancement of oxidative stabilization of viscose rayon fibers impregnated with ammonium sulfate prior to carbonization and activation steps. J Appl Polym Sci 128:1239–1249. https://doi.org/10.1002/app.38496
Rahman MM, Demirel T, Tunçel KŞ, Karacan I (2021) The effect of the ammonium persulfate and a multi-step annealing approach during thermal stabilization of polyacrylonitrile multifilament prior to carbonization. J Mater Sci 56:14844–14865. https://doi.org/10.1007/s10853-021-06209-1
Hindeleh AM, Johnson DJ, Montague PE (1980) Computational methods for profile resolution and crystallite size evaluation in fibrous polymers. ACS Publications, Washington, DC, pp 149–182
Hindeleh AM, Johnson DJ (1978) Crystallinity and crystallite size measurement in polyamide and polyester fibres. Polymer (Guildf) 19:27–32. https://doi.org/10.1016/0032-3861(78)90167-2
Yu M-J, Bai Y-J, Wang C-G et al (2007) A new method for the evaluation of stabilization index of polyacrylonitrile fibers. Mater Lett 61:2292–2294. https://doi.org/10.1016/j.matlet.2006.08.071
Stokes AR (1948) A numerical Fourier-analysis method for the correction of widths and shapes of lines on X-ray powder photographs. Proc Phys Soc 61:382–391. https://doi.org/10.1088/0959-5309/61/4/311
Arseneau DF (1971) Competitive reactions in the thermal decomposition of cellulose. Can J Chem 49:632–638. https://doi.org/10.1139/v71-101
Shafizadeh F, Bradbury AGW (1979) Thermal degradation of cellulose in air and nitrogen at low temperatures. J Appl Polym Sci 23:1431–1442. https://doi.org/10.1002/app.1979.070230513
Mwaikambo LY, Ansell MP (2002) Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J Appl Polym Sci 84:2222–2234. https://doi.org/10.1002/app.10460
Kabir MM, Islam MM, Wang H (2013) Mechanical and thermal properties of jute fibre reinforced composites. J Multifunct Compos 1:71–77. https://doi.org/10.12783/issn.2168-4286/1.1/Islam
Sharma HSS, van Sumere CF (1992) The Biology and processing of flax. M Publications, Belfast
Thuault A, Eve S, Blond D et al (2014) Effects of the hygrothermal environment on the mechanical properties of flax fibres. J Compos Mater 48:1699–1707. https://doi.org/10.1177/0021998313490217
Kondo T (1997) The assignment of IR absorption bands due to free hydroxyl groups in cellulose. Cellulose 4:281–292. https://doi.org/10.1023/A:1018448109214
Khan MA, Hassan MM, Drzal LT (2005) Effect of 2-hydroxyethyl methacrylate (HEMA) on the mechanical and thermal properties of jute-polycarbonate composite. Compos Part A Appl Sci Manuf 36:71–81. https://doi.org/10.1016/j.compositesa.2004.06.027
Tsuboi M (1957) Infrared spectrum and crystal structure of cellulose. J Polym Sci 25:159–171. https://doi.org/10.1002/pol.1957.1202510904
Carrillo F, Colom X, Valldeperas J et al (2003) Structural characterization and properties of lyocell fibers after fibrillation and enzymatic defibrillation finishing treatments. Text Res J 73:1024–1030. https://doi.org/10.1177/004051750307301114
Fanti G, Baraldi P, Basso R, Tinti A (2013) Non-destructive dating of ancient flax textiles by means of vibrational spectroscopy. Vib Spectrosc 67:61–70. https://doi.org/10.1016/j.vibspec.2013.04.001
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J Appl Polym Sci 8:1325–1341. https://doi.org/10.1002/app.1964.070080323
Liang CY, Marchessault RH (1959) Infrared spectra of crystalline polysaccharides. I. Hydrogen bonds in native celluloses. J Polym Sci 37:385–395. https://doi.org/10.1002/pol.1959.1203713209
Roy A, Chakraborty S, Kundu SP et al (2012) Improvement in mechanical properties of jute fibres through mild alkali treatment as demonstrated by utilisation of the Weibull distribution model. Bioresour Technol 107:222–228. https://doi.org/10.1016/j.biortech.2011.11.073
Dadashian F, Yaghoobi Z, Wilding MA (2005) Thermal behaviour of lyocell fibres. Polym Test 24:969–977. https://doi.org/10.1016/j.polymertesting.2005.08.005
Abdelmouleh M, Boufi S, Belgacem MN et al (2004) Modification of cellulosic fibres with functionalised silanes: development of surface properties. Int J Adhes Adhes 24:43–54. https://doi.org/10.1016/S0143-7496(03)00099-X
Acknowledgements
This study was supported by a grant from the Higher Education Council of Turkey to Md. Mahbubor Rahman under the YÖK Scholarship Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest among the authors.
Additional information
Handling Editor: Stephen Eichhorn.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahman, M.M., Karacan, I. The impact of eco-friendly chemical incorporation on the thermal oxidation process of flax fiber prior to carbonization and activation. J Mater Sci 57, 2318–2333 (2022). https://doi.org/10.1007/s10853-021-06686-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06686-4