Abstract
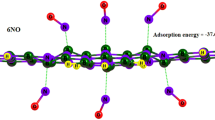
Two-dimensional (2D) monolayers have opened a new door for further studies in search of multifunctional materials. In addition to the interesting properties that these monolayers exhibit on their own, these properties can be tuned by doping, creating defects or adatom adsorption processes. Recent research has focused on determining in what technological areas these monolayers can be used. Boron carbide (BC) is a new single layer material that has been shown to be stable from the boron-doped graphene family, but its uses, such as its sensing, uptaking and fixation ability of toxic gases, have not been fully determined yet. This study is a step taken in order to fill the gap in this field. Adsorption of CO and NO molecules on the BC monolayer has been investigated by using first principles DFT methods. After structural optimization, the adsorption energies have been computed for the model systems. Electronic properties of stable structures have been determined by introducing the total and partial DOS plots and charge distributions. Our results revealed that BC monolayer successfully adsorbed CO and NO toxic gases. Thus, useful information was provided for possible applications of a base material, such as detection, uptake, and fixation of toxic gases.









Similar content being viewed by others
Change history
27 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10853-021-06550-5
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Bolotin KI, Sikes KJ, Jiang Z, Klima M, Fudenberg G et al (2008) Ultrahigh electron mobility in suspended graphene. Solid State Commun 146:351–355
Lee C, Wei X, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321:385–388
Schwierz F (2010) Graphene transistors. Nat Nanotechnol 5:487–496
Yoon HJ, Jun DH, Yang JH, Zhou Z (2011) Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem 157:310–313
Singh E, Meyyappan M, Nalwa HS (2017) Flexible graphene-based wearable gas and chemical sensors. ACS Appl Mater Interfaces 9:34544–34586
Bonaccorso F, Sun Z, Hasan T, Ferrari AC (2010) Graphene photonics and optoelectronics. Nat Photonics 4:611–622
Xianglong L, Linjie Z (2018) Graphene hybridization for energy storage applications. Chem Soc Rev 47:3189–3216
Kumar R, Singh R, Hui D, Feo L, Fraternali F (2018) Graphene as biomedical sensing element: state of art review and potential engineering applications. Compos B 34:193–206
Perreault F, Fonseca de Faria A, Elimelech M (2015) Environmental applications of graphene-based nanomaterials. Chem Soc Rev 44:5861–5896
Mannix AJ, Zhou X-F, Kiraly B, Wood JD, Alducin D et al (2015) Synthesis of borophenes: anisotropic, two-dimensional boron polymorphs. Science 350:1513–1516
Cahangirov S, Topsakal M, Aktürk E, Sahin H, Ciraci S (2009) Two- and one-dimensional honeycomb structures of silicon and germanium. Phys Rev Lett 102:236804
Dávila ME, Xian L, Cahangirov S, Rubio A, Le Lay G (2014) Germanene: a novel two-dimensional germanium allotrope akin to graphene and silicene. New J Phys 16:095002
Zhu Z, Tománek D (2014) Semiconducting layered blue phosphorus: a computational study. Phys Rev Lett 112:176802
Aktürk OÜ, Ongun Özçelik V, Ciraci S (2015) Single-layer crystalline phases of antimony: antimonenes. Phys Rev B 91:235446
Kamal C, Ezawa M (2015) Arsenene: two-dimensional buckled and puckered honeycomb arsenic systems. Phys Rev B 91:085423
Balendhran S, Walia S, Nili H, Sriram S, Bhaskaran M (2015) Elemental analogues of graphene: silicene, germanene, stanene, and phosphorene. Small 11:640–652
Ongun Özçelik V, Kecik D, Durgun E, Ciraci S (2015) Adsorption of group IV elements on graphene, silicene, germanene, and stanene: Dumbbell formation. J Phys Chem C 119:845–853
Li S, Wu Y, Tu Y et al (2015) Defects in silicene: vacancy clusters. Ext Line Defects Di-adatoms Sci Rep 5:7881
Sun X, Liu Y, Song Z, Li Y, Wang W, Lin H, Wang L, Li Y (2017) Structures, mobility and electronic properties of point defects in arsenene, antimonene and an antimony arsenide alloy. J Mater Chem C 5:4159–4166
Şahin H, Cahangirov S, Topsakal M, Bekaroglu E, Akturk E, Senger RT, Ciraci S (2009) Monolayer honeycomb structures of group-IV elements and III-V binary compounds: first-principles calculations. Phys Rev B 80:155453
Zhang K, Feng Y, Wang F, Yang Z, Wang J (2017) Two dimensional hexagonal boron nitride (2D-hBN): synthesis, properties and applications. J Mater Chem C 5:11992–12022
Bhauriyal P, Mahata A, Pathak B (2018) Graphene-like carbon-nitride monolayer: a potential anode material for Na- and K-ion batteries. J Phys Chem C 122:2481–2489
Coleman JN, Lotya M, O’Neill A et al (2011) Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 331:568–571
Luo B, Yao Y, Tian E, Song H, Wang X, Li G et al (2019) Graphene-like monolayer monoxides and monochlorides. PNAS 116:17213–17218
Wu S, Ren W, Xu L, Li F, Cheng H-M (2011) Doped graphene sheets as anode materials with superhigh rate and large capacity for lithium ion batteries. ACS Nano 5:5463–5471
Wang X, Zeng Z, Ahn H, Wang G (2009) First-principles study on the enhancement of lithium storage capacity in boron doped graphene. Appl Phys Lett 95:183103
Wang H, Zhang C, Liu Z, Wang L, Han P, Xu H, Zhang K, Dong S, Yao J, Cui G (2011) Nitrogen-doped graphenenanosheets with excellent lithium storage properties. J Mater Chem 21:5430–5434
Torres AE, Flores R, Fomina L, Fomine S (2016) Electronic structure of boron-doped finite graphene sheets: unrestricted DFT and complete active space calculations. Mol Simul 42:1512–1518
Agnoli S, Favaro M (2016) Doping graphene with boron: a review of synthesis methods, physicochemical characterization, and emerging applications. J Mater Chem A 4:5002–5025
Yanagisawa H, Tanaka T, Ishida Y, Matsue M, Rokuta E, Otani S, Oshima C (2004) Phonon dispersion curves of a \(BC_3\) honeycomb epitaxial sheet. Phys Rev Lett 93:177003
Luo X, Yang J, Liu H, Wu X, Wang Y, Ma Y, Wei S-H, Gong X, Xiang H (2001) Predicting two-dimensional boron-carbon compounds by the global optimization method. J Am Chem Soc 133:16285–16290
Zhou L, Gao J, Liu Y, Liang J, Javid M, Shah A, Dong X, Yu H, Quan X (2019) Template synthesis of novel monolayer \(B_{4}C\) ultrathin film. Ceram Int 45:2909–2916
Nersisyan HH, Yoo BU, Joo SH, Lee TH, Lee KH, Lee JH (2015) Polymer assisted approach to two-dimensional (2D) nanosheets of \(B_{4}C\). Chem Eng J 281:218–226
Ling C, Mizuno F (2014) Boron-doped graphene as a promising anode for Na-ion batteries. Phys Chem Chem Phys 16:10419–10424
Das D, Hardikar RP, Han SS, Lee K-R, Singh AK (2017) Monolayer \(BC-2\): an ultrahigh capacity anode material for Li ion batteries. Phys Chem Chem Phys 19:24230–24239
Gong S, Wang Q (2017) Boron-doped graphene as a promising anode material for potassium-ion batteries with a large capacity, high rate performance, and good cycling stability. J Phys Chem C 121:24418–24424
Ji S, Zhao J (2018) Boron-doped graphene as a promising electrocatalyst for NO electrochemical reduction: a computational study. New J Chem 42:16346–16353
Velázquez-López L-F, Pacheco-Ortin S-M, Mejía-Olvera R, Agacino-Valdés E (2019) DFT study of CO adsorption on nitrogen/boron doped-graphene for sensor applications. J Mol Model 25:91
Rad AS, Shabestari SS, Mohseni S, Aghouzi SA (2016) Study on the adsorption properties of \(O_3\), \(SO_2\), and \(SO_3\) on B-doped graphene using DFT calculations. J Solid State Chem 237:204–210
Dai J, Yuan J, Giannozzi P (2009) Gas adsorption on graphene doped with B, N, Al, and S: a theoretical study. Appl Phys Lett 95:232105
Schedin F, Geim AK, Morozov SV, Hill EW, Blake P, Katsnelson MI, Novoselov KS (2007) Detection of individual gas molecules adsorbed on graphene. Nat Mater 6:652–655
Mahabal MS, Deshpande MD, Hussain T, Ahuja R (2015) Sensing characteristics of a graphene-like boron carbide monolayer towards selected toxic gases. Chem Phys Chem 16:3511–3517
Aghaei SM, Monshi MM, Torres I, Zeidi SMJ, Calizo I (2018) DFT study of adsorption behavior of NO, CO, \(NO_2\), and \(NH_3\) molecules on graphene-like \(BC_3\): a search for highly sensitive molecular sensorApplied. Surf Sci 427:326–333
Naqvi SR, Hussain T, Gollu SR, Luo W, Ahuja R (2020) Superior sensitivity of metal functionalized boron carbide (\(BC_3\)) monolayer towards carbonaceous pollutants. Appl Surf Sci 512:145637
Tang Y, Cui X, Chen W, Zhu D, Chai H, Dai X (2018) A theoretical study on metal atom-modified \(BC_3\) sheets for effects of gas molecule adsorptions. Appl Phys A 124:434
Yeganeh M, Saraf HH, Kafi F, Boochani A (2020) First principles investigation of vibrational, electronic and optical properties of graphene-like boron carbide. Solid State Commun 305:113750
Mishra P, Singh D, Sonvane Y, Ahuja R (2020) Excitonic effects in the optoelectronic properties of graphene-like BC monolayer. Opt Mater 110:110476
Kresse G, Furthmuller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B Condens Matter Mater Phys 54:11169
Kresse G, Furthmuller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B Condens Matter Mater Phys 50:1758
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 6:6671
Monkhorst HJ, Pack JD (1976) Special points for brillouin-zone integrations. Phys Rev B 13:5188
Broyden CG (1970) The Convergence of a class of double-rank minimization algorithms 1. General considerations. J Inst Math Appl 6:76–90
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44:1272–1276
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I, Dal Corso A, Fabris S, Fratesi G, de Gironcoli S, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen AP, Smogunov A, Umari PRM, Wentzcovitch RM (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys Condens Matter 21(395502):395502
Wang W, Zhang Y, Shen C, Chai Y (2016) Adsorption of CO molecules on doped graphene: a first-principles study. AIP Adv 6:025317
Zhang Y-H, Chen Y-B, Zhou K-G, Liu C-H, Zeng J, Zhang H-L, Peng Y (2009) Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study. Nanotechnology 20:185504
Leenaerts O, Partoens B, Peeters FM (2008) Adsorption of \(H_{2}O\), \(NH_3\), CO, \(NO_2\), and NO on graphene: a first-principles study. Phys Rev B 77:125416
Gao H, Liu Z (2017) DFT study of NO adsorption on pristine graphene. RSC Adv 7:13082–13091
Cai Y, Ke Q, Zhang G, Zhang Y-W (2015) Energetics, charge transfer, and magnetism of small molecules physisorbed on phosphorene. J Phys Chem C 119:3102–3130
Lei S, Gao R, Sun X, Guo S, Yu H, Wan N, Xu F, Chen J (2019) Nitrogen-based gas molecule adsorption of monolayer phosphorene under metal functionalization. Sci Rep 9:12498
Jahn HA, Teller E (1937) Stability of polyatomic molecules in degenerate electronic states - I-Orbital degeneracy. Proc R Soc London A 161:220–235
Manz TA, Limas NG (2016) Introducing DDEC6 atomic population analysis: part 1. Charge Partit Theory Methodol RSC Adv 6:47771–47801
Limas NG, Manz TA (2016) Introducing DDEC6 atomic population analysis: part 2. Computed results for a wide range of periodic and nonperiodic materials. RSC Adv 6:45727–45747
Manz TA (2017) Introducing DDEC6 atomic population analysis: part 3. Comprehensive method to compute bond orders. RSC Adv 7:45552–45581
Mills G, Jonsson H, Schenter GK (1995) Reversible work transition state theory: application to dissociative adsorption of hydrogen. Surf Sci 324:305–337
Henkelman G, Jonsson H (2000) Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J Chem Phys 113:9978–9985
Henkelman G, Uberuaga BP, Jonsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys 113:9901–9904
Sheppard D, Xiao P, Chemelewski W, Johnson DD, Henkelman G (2012) A generalized solid-state nudged elastic band method. J Chem Phys 136:074103
Acknowledgements
Computing resources used in this work were provided by the TUBITAK (The Scientific and Technical Research Council of Turkey) ULAKBIM, High Performance and Grid Computing Center (Tr-Grid e Infrastructure). CK also acknowledged the scientific collaboration with the TARLA project funded by the Presidency of Turkey, Strategy and Budget Department Grant No: 2006K12-827. HA and EA acknowledge support from Alexander von Humboldt Foundation (Germany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Handling Editor: Yaroslava Yingling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to correct the designation of author affiliations.
Rights and permissions
About this article
Cite this article
Kaderoglu, C., Akturk, E. & Arkin, H. High uptake and fixation ability of BC monolayer for CO and NO toxic gases: a computational analysis. J Mater Sci 56, 18566–18580 (2021). https://doi.org/10.1007/s10853-021-06524-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06524-7