Abstract
Although metals have successfully been used as implants for decades, devices made out of metals do not meet all clinical requirements. For example, metal objects may interfere with some medical imaging systems (computer tomography, magnetic resonance imaging), while their stiffness also differs from natural bone and may cause stress-shielding and over-loading of bone. There has been a lot of development in the field of composite biomaterial research, which has focused to a large extent on biodegradable composites. This overview article reviews the rationale of using glass fiber-reinforced composite–bioactive glass (FRC–BG) in cranial implants. For this overview, published scientific articles with the search term “bioactive glass cranial implant” were collected for having basis to introduce a novel design of composite implant, which contains bioactive glass. Additional scientific information was based on articles in the fields of chemistry, engineering sciences and dentistry. Published articles of the material properties, biocompatibility and possibility to add bioactive glass to the FRC–BG implants alongside with the clinical experience as far suggest that there is a clinical need for bioactive nonmetallic implants. In the FRC–BG implants, biostable glass fibers are responsible for the load-bearing capacity of the implant, while the dissolution of the bioactive glass particles supports osteogenesis and vascularization and provides antimicrobial properties for the implant. Material combination of FRC–BG has been used clinically in cranioplasty and cranio-maxillo-facial implants, and they have been investigated also as oral and orthopedic implants. Material combination of FRC–BG has successfully been introduced to be a potential implant material in cranial surgery.
Similar content being viewed by others
Introduction
It can be estimated that worldwide over 2 million bone graft procedures, 280,000 hip fractures, 700,000 vertebral, 250,000 wrist fractures and 700,000 various cranial bone repairs are annually performed [1]. In particular, the need for skull reconstructions, i.e., cranioplasties, is increasing mainly due to an increase in decompressive craniectomies, a life-saving maneuver to relieve intracranial pressure resulting from swelling of the brain due to, for example, trauma or cerebrovascular accidents. Replacement of damaged tissues by medical biomaterials after an injury or disease requires specific properties from the materials. There is an increasing trend to utilize nonmetallic materials of polymers, ceramics and composites rather than metals although metals are durable and can withstand physiological stress relatively well. Although metal implants have been used successfully for many years, devices made out of metals do not meet all biomechanical requirements, such as isoelasticity of skeleton and bone, and may lead to insufficient (stress-shielding) or over-loading situations around the implant [2]. This problem has been recognized specifically when used as metal implants in long bones as total hip replacement implants but in reconstructions of segmental defects of mandible, lack of isoelasticity may play a role too. Metal implants may also induce cytotoxic reactions arising from the release of metal ions, corrosion products and nanoparticles [3,4,5]. Potential cytotoxicity arising from heavy metal ion liberation and harmful corrosion products and nanoparticles are suggested to be harmful for the immunological system of human body, which in the case of released Ti4+ ions are causing soft tissue atrophy and potentially exposure of the implants [6]. In addition, although the most commonly used titanium is not magnetic metal, all metallic objects interfere with medical diagnostics when using computer tomography, magnetic resonance imaging (MRI) and cone beam X-ray imaging [7,8,9]. Metals do not allow postoperative radiation therapy to be performed either due to absorption and scattering of the radiation.
Biodegradable and biostable medical composite materials have been developed considerably in recent decades [10]. Currently, they can be used in some applications in reconstructive medicine. Although numerous different materials, such as polyethylene (PE), polymethylmethacrylate (PMMA) and polyetheretherketone (PEEK) and techniques, have been and are under investigation, there is not yet the perfect solution for bone reconstruction because large number of infections relate to autologous bone flaps and implants of various materials [9,10,11,12]. Paradoxically, when the metals are radiologically considered too dense materials, polymers of pf PE, PMMA and PEEK are having disadvantage of being radiolucent, which means that that the material cannot be seen either by conventional X-rays, CTs or MRI images [11].
Bulk ceramic biomaterials of hydroxyapatite (HA) and tricalciumphosphate (TCP) have also been tested as cranial implants [12]. Brittleness of the ceramic materials especially when the material has been processed to porous form is a limiting factor for the clinical use of ceramic materials. Brittleness and low strength have tried to be resolved by reinforcing the ceramic with metallic titanium [13].
Durable and tough nonmetallic composites can be made from high-aspect-ratio fillers, namely fibers embedded in a polymer matrix. The first studies using fiber-reinforced composites (FRCs) in medicine and dentistry occurred in the early 1960s, but more extensive research started in the early 1990s which led to introduction of FRCs as reconstructive material for damaged dental hard tissues [14,15,16,17,18]. The first approved surgical applications were found in cranial surgery [18]. To improve osteoconductivity and osteogenicity of the FRC material, particles of bioactive glass have been added to the surface of FRC implants or inside the implant [19,20,21,22,23]. Radiopacity of glass FRC corresponds to that of cortical bone, and therefore there are no artefacts in the diagnostic images, but the implant can be seen in the X-rays, CTs and MRIs (Fig. 1) [24]. Radiation therapy can also be given in the presence of FRC implant. This overview describes the present status of the development and use of nonmetallic predominantly biostable glass FRC–BG implants with special emphasis on cranial bone replacing implants. Table 1 lists properties of cranial implant materials with respect to their clinically needed properties.
Consequently, because of the need for cranial implants, which are nonmetallic and bioactive, a potential material to be used in the cranial implants is bioactive glass (BG). A review of published scientific articles in PubMed (US National Library of Medicine, National Institutes of Health, Bethesda, Maryland, USA) with a search word “bioactive glass cranial implant” that found 45 publications was the basis for this overview article. Additional scientific information was included to this overview from other fields of sciences, namely from chemistry, engineering sciences and dentistry.
Implant framework
For constructing a durable and nonmetallic implant, the material should be high in strength (flexural, impact and tensile strength) and provide good fracture propagation prohibiting properties (toughness). To reach these mechanical properties, FRC material consisting of high-aspect ratio reinforcing fibers and polymer material were used. Presently, the most commonly used reinforcing fibers in medical and dental field are made of glass of various compositions [29,30,31,32,33,34,35]. Glass fibers referred as E-glass and S-glass are basically free of leaching in physiologically moist environment like in living tissues with the presence of extracellular liquid. Nominal composition (in wt%) of commonly used E-glass is SiO2 55; Al2O3 + Fe2O3 14.5; CaO 21.5; MgO 0.5%; Na2O + K2O < 1.0; B2O3 7.5, and for S-glass SiO2 62–65; Al2O3 20-25; MgO 10–15; B2O3 0–1.2; Na2O 0–1.1; Fe2O3 0.2.
Glass fibers of diameter 15–17 micrometers are used in implants as continuous fibers which have been woven to textile form. Woven fibers (i.e., bidirectional continuous fiber system) of the FRC material divide the reinforcing effect into the two directions, which are the directions of the fibers. If the fiber structure is made of unidirectional continuous fibers only, the maximal reinforcing effect (Krenchel’s factor 1) can be obtained [34, 36]. In the presently used design of FRC cranial implants, both woven textile form fibers and unidirectional fibers are used in the implant construction (Fig. 2) [37]. Combination of the two kinds of fiber systems allows designing a sandwich structure for the implant with mesh-like outer and inner surface laminates of the implant. Inner and outer FRC laminates are connected to each other by additional continuous unidirectional FRC bars, which connect the laminates together and provide high-strength reinforcing element to the implant. Depending on the implant size and strength requirements, the implant can contain one or several unidirectional FRC bars in the construction. Special features of the FRC cranial implant construction are mesh-like surface laminate and presence of free space between the outer and inner laminates, which is loaded with bioactive modifiers, i.e., particles of bioactive glass (Fig. 3) [37].
Polymer matrix of FRC material binds the biostable reinforcing fibers together and protects the fibers. When FRC construction is loaded, stress is transferred from resin matrix to be carried by the reinforcing fibers with specific orientation [34]. During transferring the load from the polymer matrix to the stronger fibers, a durable adhesion between the reinforcing fibers and the polymer matrix is needed. In the case of glass fibers with hydroxyl group covered surface, silane coupling agents are used for improving quality of the adhesive interface [38,39,40,41,42]. Resins are thermoplastics, thermosets or their combinations in the form of semi-interpenetrating polymer networks (semi-IPN). Examples of thermoplastics used in implants are polyethylene (PE), polyetheretherketone (PEEK). Examples of thermosets which are utilized as medical biomaterials are epoxy polymers and bisglycidyl-A-dimethacrylate (BisGMA), triethylene glycol dimethacrylate (TEGDMA) and urethanedimethacrylate (UDMA). Thermosets which are polymerized from the monomers in the presence of silanized glass fibers form durable chemical adhesion to the glass fibers, whereas thermoplastics are only physically interlocked to the surface of fibers [42]. For this reason, dimethacrylate monomers have been selected to be used in the FRC–BG cranial implants. In the FRC with PEEK polymer, the fibers are only physically attached to the polymer matrix.
Polymerization reaction of monomer systems, which forms thermoset polymers, is based of free radical (vinyl) polymerization. Initiation of the polymerization is made by autopolymerization or radiation of blue light with wave length of 463 nm [34]. Typically, the autopolymerization is initiated by peroxide-amine system and the light-initiated polymerization is based on initiator system of camphorquinone–amine system. Thermoset polymers can be post-cured by heat after initial curing which increases considerably the degree of monomer conversion, reduces quantity of residual monomers and improves biocompatibility [43,44,45,46]. Optimal post-curing temperature is close to the glass transition temperature where there is enough thermal energy in the system to create free volume, which enables unreacted carbon–carbon double bonds to form free radicals and react with each others [47].
Long-term structural success of the reconstructive composite materials in biological environment depends to large extent on the hydrolytic stability of the composite. Hydrolytic stability is dependent on the stability of polymer matrix, stability of fillers and stability of the interface between fillers and polymer matrix. Presently used glass FRC exhibit good long-term hydrolytic stability, which is based on the stability of thermoset polymer matrix and glass fibers and their interface [39, 42, 48]. It is known that good-quality and surface-purified glass fibers itself exhibit stability in pH between 3 and 10, meaning that the pH of tissues in normal and pathological conditions do not considerably leach the glass fibers and glass fibers can be considered biostable material in vivo [49].
Continuous unidirectional FRC, which is used in the load-bearing part of the FRC–BG implant, has flexural strength of 1200 MPa, whereas the mesh-like FRC laminate of the outer and inner surface is having strength of 400–600 MPa due to lower reinforcing efficiency factor (Krenchel’s factor) by the bidirectionally directed fibers [50, 51]. It needs to emphasize that the load-bearing capacity for the implant structure comes from the FRC material properties and from the sandwich structure of the implant, its shape, its initial screw fixation and finally from the osseointegration and bone ingrowth. When the implant of this design is loaded, ductile-type fracture occurs, i.e., continuous glass fibers do not break although laminates delaminate from each other. Bending deformation of the magnitude of 10 mm with a typical-sized cranial implant is still within the area of elastic deformation, and the implant receives its original shape after releasing the external force. Load-bearing capacity of the FRC–BG implant with size of 112 × 67 mm after being fixed and osseointegrated in the simulated conditions reached fracture force of 649 N. Resistance of glass fibers, polymer matrix and their adhesive interface has been shown to be good in long-term in vitro studies during the time of ten years and in vivo with the follow-up time of more that two years. However, the FRC material is weakened by ca. 15% during the first one month time being in water-containing environment due to plasticization effect of the polymer matrix, but reduction of strength does not continue in coming years of storing the material in water-containing solutions [37, 48].
Biocompatibility of FRC material
Biocompatibility of FRC implants is basically related to the biocompatibility of its major components of polymer matrix, reinforcing glass fibers and bioactive glass. Thermoset polymer FRC has been made of dimethacrylate resin systems but in some cases also of epoxy resins. Use of epoxy polymer has been criticized due to potential toxic and allergic effects of its monomers, which are present as residuals in the FRC [46, 47, 52]. On the other hand, thermoset polymers made of dimethacrylate monomer systems of BisGMA have shown good biocompatibility after careful polymerization before insertion of the material to tissues [53, 54] However, when the BisGMA monomers are allowed to polymerize in situ, for example, as bone cement, the biocompatibility of the cement has been questioned [55, 56].
Biological testing of glass FRC by cell culture and animal testing have shown material’s biocompatibility. Cell culture study by fibroblasts with silanized E-glass fibers without the resin matrix has shown no signs of cytotoxicity, as it has been also demonstrated with fibroblasts by agar diffusion cytotoxicity test and animal experiments [30, 56,57,58,59,60,61,62]. In the form of FRC implant, glass fibers are covered by the thermoset polymer matrix and the only areas where the glass fibers are exposed are located at the margins of the implant which have been finished mechanically or by laser ablation. By using osteoblasts on the cell culture model with FRC implants, no signs of toxic reactions of the material were found. For instance, when bone marrow-derived osteoblast-like cells were harvested and cultured on the FRC material plates and on commercially pure titanium plates and cell growth and differentiation kinetics were investigated, similar alkaline phosphatase activities on both FRC and titanium were observed [62, 63]. Expression of osteoblastic markers of osteocalcin and bone sialoprotein indicated that the fastest osteogenic differentiation took place on FRC after 7 days. In contrast, a slower differentiation process was observed on titanium. It was concluded that the proliferation and maturation of osteoblast-like cells on FRC appeared to be comparable to titanium. Presence of BG on the implant surface enhanced cell maturation.
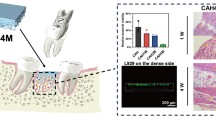
A number of preclinical animal experiments have been carried out to show cell response to FRC in vivo. In many of the FRC material studies, there have been additional BG (S53P4) particles on the surface of the FRC implant [24, 25, 62, 63]. BGs are synthetic resorbable, biocompatible, osteoconductive–osteoinductive bone substitutes, and some compositions of BGs have clinically been used because of bone-bonding capacity, antibacterial and angiogenesis-promoting properties [64,65,66,67,68,69]. FRC–BG implant has been tested by animal tests for cranial implant applications as well as for orthopedics and oral implantology. Animal experiments with cranial implant applications have been made with calvarial critical size defect model with rabbits with implants having lamellar FRC structure [30, 59, 60]. Between the laminates of the implant, there were particles of bioactive glass for improving osteogenesis, angiogenesis and antimicrobial properties. Rabbit experiments with newly cut critical size defects showed new maturating bone ingrowth into the implant through holes on the implant surface (Fig. 4).
Scanning electron micrograph of the surface of FRC–BG implant after in vitro simulated body fluid testing showing a surface of fiber-reinforced composite, b leaching particle of bioactive glass and c biomineralization layer of the implant surface (original magnification ×30). Histological images (HE staining) show new-forming bone ingrowth to the implant (upper image) [60] bone contact to the surface of the fiber-reinforced composite (lower image) [62]
FRCs have the potential for the use as load-bearing orthopedic implants as well. An experimental animal study was carried out to test the in vivo performance of glass FRC implants made of unidirectional glass fibers and BG (S53P4) surface coverage [24, 29]. Control implants were made of surface-roughened titanium. Stress-shielding effects of the implants were predicted by finite element modeling (FEM) [24, 70]. Surgical stabilization of bone metastasis in the subtrochanteric region of the femur was simulated in a rabbit model. An oblong subtrochanteric defect of a standardized size (reducing the torsional strength of the bones approximately by 66%) was created, and an intramedullary implant made of titanium or the FRC–BG was inserted. The contralateral femur served as the intact control. After healing, the femurs were harvested and analyzed. The functional recovery was unremarkable in both groups. FEM studies demonstrated differences in stress-shielding effects of the titanium and FRC implants: FRC implants had bone-like biomechanical properties. The torsional strength of the fixed bones had returned the level of contralateral intact femurs. Oral implant research has also utilized glass FRC of BisGMA and TEGDMA polymer matrix system in studies with experimental animals. The studies have also shown FRC–BG implant’s biocompatibility in bone to be comparable to that of titanium. Addition of BG to the implant surface increased contact of bone to the implant and bone maturation [61,62,63].
Bioactive glass used in cranial implants
Out of several compositions and particle sizes of bioactive glass, clinically the most potential bioactive glass in bone augmentation indications is silicate glass S53P4 with the nominal composition (in wt %) of Na2O 23; CaO 20; B2O5 4; SiO2 53, and average particle size on 500 μm [71]. Leaching of BG and the released ions are behind the biological function of the glass, and detailed knowledge of these reactions is a key to selecting BGs as component in implants. BG S53P4 has shown to fulfill several known requirements for osteogenesis and bone remodeling.
Biological function of BG is twofold: release of ions of calcium and phosphorus is causing biomineralization on the bioactive material surface, like on the surface of glass FRC and extracellular matrix of new-forming bone. For cells, at the early stage of osteogenesis, released ions from the BG and slightly increased pH due to ion exchange reactions are inducing differentiation of mesenchymal stem cells to cell lines for bone formation [68]. This, in conjunction with biomineralization promotes bone growth. It is essential to understand the microenvironment where cell differentiation occurs. If the pH increases too much due to ion exchange by the BG, differentiation of cells does not happen and cells can eventually die. Too high increase in pH can be because of inadequate flow of interstitial liquid, too small particle size of BG and too reactive leaching profile of BG due to its composition [72]. Level of pH where differentiation of mesenchymal stem cells is hindered is around 8.5, whereas the effective differentiation can be seen in pH of 7.8–8.0 [68, 72]. There is also in vitro obtained information that BG can induce vascularization, and indeed, histological analysis of new bone around BG shows the presence of blood vessels [37, 68, 73].
With regard to osseointegration, i.e., bonding between the BG of the implant and tissue, a series of reactions starting at the glass surface followed by a series of biological reactions are occurring. The different reaction steps taking place at the glass surface depend mainly on the glass composition but also on the surface topography, surface area of glass, and flow of the interstitial fluid in the microenvironment close to the glass surfaces. In the subsequent steps, calcium and phosphate from the solution, and migrating from the bulk glass, form first amorphous hydroxyapatite and then crystallize at carbonate substituted hydroxyapatite layer (HA) at the glass surface (Fig. 3). This HA layer is compatible with the biological apatite and provides an interfacial bonding between the material and tissue.
Antibacterial properties of the glasses are attributed to the local rise of pH level and increased ion concentration causing increased osmotic pressure [74]. The US Food and Drug Administration (FDA) approved BG 45S5 and BG S53P4 for certain clinical applications where antimicrobial properties are required. Increase in the alkalinity by bioactive glass 45S5 is higher than by glass S53P4, and therefore glass 45S5 is considered to be more effective in terms of antimicrobial properties. On the other hand, a balance between antimicrobial properties, i.e., increase in pH and moderate alkalinity and ion release and osteogenicity, has been found with BG S53P5. In vitro conditions in the presence of BG S53P4 showed the increase in pH to the level of 7.9 [35]. Antimicrobial efficiency has been shown for more than 20 microbe species, including Staphylococcus aureus and Staphylococcus epidermis, which are the most common pathogens in periprosthetic infections [75, 76]. Antimicrobial properties have been beneficial also in augmentation of bone defects which are prone for infections [77, 78].
Clinical use and development stages of FRC–BG implants
To overcome discomfort and pain by cranial and facial bone reconstructions based on autologous bone transplants, and problems related to biomaterial implants, patient-specific FRC–BG cranial implants were started to be used first time in 2007 [23]. Before the time FRC–BG implants, the first-generation implants were made of bulk polymethylmethacrylate (PMMA) which has been polymerized ex vivo and covered from the surface with exposing particles of BG S53P4 [79]. Based on the clinical experiences with the PMMA implants, further improvements in terms of allowing osteogenesis and vascularization to occur inside the implant and to have thinner and cosmetically more pleasant looking margins for the implants, studies of FRC–BG implants started [30, 59, 60, 80, 81].
The first FRC–BG implants were loaded with BG S53P4 and the implant structure had dense outer and inner surface laminates made of glass FRC fabric and between the layers there was porous glass FRC particles of BG. Implant design allowed blood penetration only by capillary forces from the sides of the implant to occur, and therefore only ca. 15 mm from the margin of the implant became in contact with blood [28]. Postoperative positron emission computer tomography (PET-CT) examination with (18F)-fluoride marker has demonstrated activity of the mineralizing bone by osteoblasts, especially at the margins of the implant into which the blood was penetrated by capillary forces (Fig. 5). When implant of that kind had been analyzed more in detail after being in situ for two years and three months, 3D CT reconstructions demonstrated ossification on the lover surface of implant which was considered as peridural ossification (Fig. 6). Histological analysis showed blood vessels and clusters of osteoblasts along the collagenous fibers with osteoid formation and clusters of bone-like hard tissue. Osteoblasts were also found on the surface of the implant with osteoid production. However, this implants design with blood penetration only to the marginal area of the implant showed the biological activity on the implant margins only, which emphasized importance of the blood penetration into the implant. Clinical follow-up study of this type of FRC–BG implant showed higher survival estimates than for other implant materials and autologous bone in of retrospective study material (Fig. 7) [81].
Positron emission tomogram with fluoride marker showing the FRC–BG implant (block arrow), margins of the implant (white arrows), margins of the original bone defect (dotted lines) and histological section (HE staining) of the osteoblasts inside the implant which had absorbed blood during the surgical operation to install the implant [37]
Three year survival of cranial bone defect reconstructions with FRC–BG implants, autologous bone flaps and other implant materials [modified from 82]
Based on the observations of the first-stage FRC–BG implants, the implant design was changed to be more mesh-like in structure. Change in the design was made for having better interstitial liquid perfusion through the implant by pulsatile movement of dura mater, which facilitated stem cells and growth factors from the refreshed bone margins at the operation site to penetrate into the implant and become in contact to BG particles, and promote osteogenesis. Recent data indicate that shear stress and circumferential stretch by pulsatile flow affects mesenchymal stem cell differentiation toward endothelial linea. Release of ions and related increase in pH by the BG enhanced osteogenesis and vascularization to occur in the implant and make the implant microenvironment bacteriostatic. For instance, phosphate ions have shown to have an important role in osteogenesis [82]. Interestingly, BG S53P4 shows higher release of phosphate ions than BG 45S5 [35], which may be one factor together with the only moderate increase of pH behind the good clinical function of the BG S53P4 compared to BG 45S5. Mechanical strength for the implant was obtained from the biostable glass FRC laminates of inner and outer surfaces of the implants and continuous unidirectional glass FRC bars which connected laminates to each other and provided space for BG particles. The present design of FRC–BG cranial implant has received good acceptance by the surgeons, and it was approved for clinical use as patient-specific implant and standard-shaped implant in Europe in 2014.
Future trends
There is a trend toward nonmetallic load-bearing implants in all fields of bone surgery. In cranial implantology, the driving forces for nonmetallic implant are requirements of medical imaging systems, requirements of radiation therapy and need to decrease number of periprosthetic infections and infections of resorbing autologous bone flaps. In the implant applications of long bones, namely in orthopedics and traumatology, driving forces are in need to eliminate stress-shielding and fatigue failures of implants. Glass FRC materials are fulfilling requirements of mechanical strength and biomechanical matching to the properties of bone, and at the same time allowing bioactive modification by presence of BG in the implant have proven to be potential material bone surgery. It looks that the development of the implant materials and implant constructions is going on the track of bioactive composites with high-aspect-ratio fillers. Considerable amount of research work has been put already on these new materials, and coming research is focusing on optimizing the biomechanical properties, function bioactive compounds and antimicrobial properties of the implants, as well as searching novel applications where bone and soft tissue applications for bioactive glasses can be combined [83,84,85,86].
References
Brydone AS, Meek D, Maclaine AS (2010) Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng Part H 224:1329–1343
Park JB, Lakes RS (1992) Biomaterials: an introduction. Plenum Press, New York
Bonfield W, Grynpas M, Tully AE, Bowman J, Abram J (1981) Hydroxyapatite reinforced polyethylene—a mechanically compatible implant material for bone replacement. Biomaterials 2:185–186
Moon EY, Yi GH, Kang JS, Lim JS, Pyo S (2011) An increase in mouse tumor growth by an in vivo immunomodulating effect of titanium dioxide nanoparticles. J Immunotoxicol 8:56–67
Latteier MJ, Berend KR, Lombardi AV, Ajluni AF, Seng BE, Adams JB (2011) Gender is a significant factor for failure of metal-on-metal total hip arthoplasty. J Arthoplasty 26:19–23
Riggio E, Chifu C, Martelli G, Ferraris C (2015) Can titanium mesh influence local recurrence management after implant-based breast reconstruction? SpringerPlus 4:482. doi:10.1186/s40064-015-1273.3
Shellock FG (2001) Metallic neurosurgical implants: evaluation of magnetic field interactions, heating, and artifacts at 1.5-tesla. J Magn Reson Imaging 14:295–299
Sawyer-Glover AM, Shellock FG (2001) MRI procedure screening: recommendations and safety considerations for biomedical implants and devices. J Magn Reson Imaging 12:92–106
Shellock FG (2002) Biomedical implants and devices: assessment of magnetic field interactions with a 3.0-tesla MR system. J Magn Reson Imaging 16:721–732
Holland SJ, Tighe BJ, Goud PLJ (1986) Polymers for biodegradable medical devices. 1. The potential of polyesters as controlled macromolecular release systems. Control Release 4:155–159
Qian Z, Fan X (2014) The application and progress of high-density porous polyethylene in repair of orbital wall defect. J Craniofac Surg 25:1451–1453
Brie J, Chartier T, Chaput C, Delage C, Pradeau B, Caire F, Boncoeur MP, Moreau JJ (2013) A new custom made bioceramic implant for the repair of large and complex craniofacial bone defects. J Craniomaxillofac Surg 41:403–407
Engstrand T, Kihlström L, Neovius E, Skogh AC, Lundgren K, Jacobsson H, Bohlin J, Åberg J, Engqvist H (2014) Development of bioactive implant for repair and potential healing of cranial defects. J Neurosurg 120:273–277
Bowers C, McMullin JH, Brimley C, Etherlington L, Siddiqi FA, Riva-Cambrin J (2015) Minimizing bone gaps when using custom pediatric cranial implants is associate with implant success. J Neurosurg Pediatr 10:1–6
Wong RK, Gandolfi BM, St-Hilaire H, Wise M, Moses M (2011) Complications of hydroxyapatite bone cement in secondary pediatric craniofacial reconstruction. J Craniofac Surg 22:247–251
Gooch MR, Gin GE, Kenning TJ, German J (2009) Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus 26:E9
Szpalsky C (2010) Cranial bone defects: current and future strategies. Neurosurg Focus 29:1–11
Vallittu PK (1999) Flexural properties of acrylic resin polymers reinforced with unidirectional and woven glass fibers. J Prosthet Dent 81(3):318–326
Vallittu PK, Sevelius C (2000) Resin-bonded, glass fiber-reinforced composite fixed partial dentures: a clinical study. J Prosthet Dent 84(4):413–418
Freilich MA, Karmarker AC, Burstone CJ, Goldberg AL (1998) Development and clinical applications of light-polymerized fiber reinforced composite. J Prosthet Dent 80:311–318
Ruyter IE, Ekstrand K, Björk N (1986) Development of carbon/graphite fiber reinforced polymethylmethacrylate suitable for implant fixed dental bridges. Dent Mater 2:6–9
Rosentritt M, Behr M, Kolbeck C, Handel G (2001) In vitro repair of three-unit fiber-reinforced composite FPDs. Int J Prosthodont 14:344–349
Aitasalo KMJ, Piitulainen JM, Rekoila J, Vallittu PK (2013) Craniofacial bone reconstruction with bioactive fiber-reinforced composite implant. Head Neck 36:722–728
Zhao DS, Moritz N, Laurila P, Mattila R, Lassila LV, Strandberg N, Mäntylä T, Vallittu PK, Aro HT (2009) Development of a multi-component fiber-reinforced composite implant for load-sharing conditions. Med Eng Phys 31(4):461–469
Goiato MC, Anchieta RB, Pita MS, Santos DM (2009) Reconstruction of skull defects: currently available materials. J Craniofac Surg 20:1512–1518
Goldstein JA, Paliga JT, Bartlett SP (2013) Cranioplasty: indications and advances. Head Neck Surg 21:400–409
Jones JR (2013) Review of bioactive glass: from Hench to hybrids. Acta Biomater 9:4457–4486
Piitulainen J (2015). Reconstruction of cranial bone defects with fiber-reinforced composite-bioactive glass implants. Turun yliopiston julkaisuja – Annales Universitatis Turkuensis Ser D-tom.1193, p 28
Moritz N, Strandberg N, Zhao DS, Mattila R, Paracchini L, Vallittu PK, Aro HT (2014) Mechanical properties and in vivo performance of load-bearing fiber-reinforced composite intramedullary nails with improved torsional strength. J Mech Behav Biomed Mater 40:127–139
Tuusa SM, Peltola MJ, Tirri T, Puska MA, Röyttä M, Aho H, Sandholm J, Lassila LV, Vallittu PK (2008) Reconstruction of critical size calvarial bone defects in rabbits with glass-fiber-reinforced composite with bioactive glass granule coating. J Biomed Mater Res B Appl Biomater 84(2):510–519
Ballo AM, Cekic-Nagas I, Ergun G, Lassila L, Palmquist A, Borchardt P, Lausmaa J, Thomsen P, Vallittu PK, Närhi TO (2014) Osseointegration of fiber-reinforced composite implants: histological and ultrastructural observations. Dent Mater 30(12):384–395
Ballo AM, Akca EA, Ozen T, Lassila L, Vallittu PK, Närhi TO (2009) Bone tissue responses to glass fiber-reinforced composite implants—a histomorphometric study. Clin Oral Implants Res 20(6):608–615
Kuusisto N, Vallittu PK, Lassila LV, Huumonen S (2015) Evaluation of intensity of artefacts in CBCT by radio-opacity of composite simulation models of implants in vitro. Dentomaxillofac Radiol 44(2):20140157
Vallittu PK (2014) High aspect ratio fillers: fiber-reinforced composites and their anisotropic properties. Dent Mater 31:1–7
Vallittu PK, Närhi TO, Hupa L (2015) Fiber glass—bioactive glass implants. Review. Dent Mater 31:371–381
Krenchel H (1963) Fibre reinforcement (Ph.D. Thesis). Copenhagen, Technical University of Denmark
Posti JP, Piitulainen JM, Hupa L, Fagerlund S, Frantzén J, Aitasalo KM, Vuorinen V, Serlo W, Syrjänen S, Vallittu PK (2015) A glass fiber-reinforced composite—bioactive glass cranioplasty implant: a case study of an early development stage implant removed due to a late infection. J Mech Behav Biomed Mater 55:191–200
Vallittu PK (1993) Comparison of two different silane compounds used for improving adhesion between fibers and acrylic denture base material. J Oral Rehabil 20:533–539
Bouillaguet S, Schutt A, Alander P, Vallittu PK, Schwaller P, Buerki G, Michler J, Cattani-Lorente M, Krejci I (2006) Influence of hydrothermal and mechanical stress to interfacial bond strength of glass fibers to polymer matrix. J Biomed Mater Res 76:98–105
Rosen MR (1978) From treating solution to filler surface and beyond. The life history of a silane coupling agent. J Coat Technol 50:70–82
Matinlinna JP, Dahl JE, Karlsson S, Lassila LVJ, Vallittu PK (2009) The effect of the novel silane system to the flexural properties of E-glass fiber reinforced composites. Silanes Other Coupling Agents 5:107–121
Matinlinna JP, Lassila LVJ, Vallittu PK (2009) Experimental novel silane system in adhesion promotion between dental resin and pretreated titanium. Silicon 1:249–254
Asmussen E, Peutzfeldt A (1998) Influence of UEDMA, BisGMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent Mater 14:51–57
Viljanen EK, Skrifvars M, Vallittu PK (2004) Degree of conversion of an experimental monomer and methyl methacrylate copolymer for dental applications. J Appl Polym Sci 93:1908–1912
Viljanen EK, Lassila LVJ, Skrifvars M, Vallittu PK (2005) Degree of conversion and flexural properties of a dendrimer/methyl methacrylate copolymer: a statistical modeling. Dent Mater 21:172–177
Mattila R, Puska M, Lassila LVJ, Vallittu PK (2006) Fibre-reinforced composite implant: in vitro interfacial failure mechanics and residual monomer analysis. J Mater Sci Mater Mater Med 41:4321–4326
Viljanen EK, Langer S, Skrifvars M, Vallittu PK (2006) Analysis of residual monomers by HPLC and HS-GC/MS. Dent Mater 22:845–851
Vallittu PK (2007) Effect of ten years of in vitro aging on the flexural properties of fiber-reinforced resin composites. Int J Prosthodont 20:43–45
Norström A, Watson H, Engström B, Rosenholm J (2001) Treatment of E-glass fibres with acid, base and silanes. Colloids Surf 194:143–157
Lassila LVJ, Tanner J, LeBell A-M, Narva K, Vallittu PK (2004) Flexural properties of fiber reinforced root canal posts. Dent Mater 20:29–36
Abdulmajeed AA, Närhi TO, Vallittu PK, Lassila LVJ (2011) The effect of high fiber fraction on some mechanical properties of unidirectional glass fiber-reinforced composite. Dent Mater 27:313–321
Tuusa SM-R, Lassila LVJ, Matinlinna JP, Peltola MJ, Vallittu PK (2005) Initial adhesion of glass-fibre reinforced composite to surface of porcine calvarial bone. J Biomed Mater Res, Part A 75:334–342
Otsuka M, Sawada M, Matsuda Y, Nakamura Y, Kokubo T (1997) Antibiotic delivery system using bioactive bone cement consisting of Bis-GMA/TEGDMA resin and bioactive glass ceramics. Biomaterials 18:1559–1564
Bae H, Hatten HP, Linovitz R, Tahernia AD, Schaufele MK, McCollom V, Gilula L, Maurere R, Benyamin R, Mathis JM, Persenaire M (2012) A prospective randomized FDA–IDE trial comparing Cortoss with PMMA for vertebroplasty: a comparative effectiveness research study with 24-month follow-up. Spine 37:544–550
Fölsch C, Pinkemell R, Stiletto R (2013) Biocompatibility of polymer-bioglass cement Cortoss: in vitro test with MG63 cell model. Orthopade 42:170–176
Ballo AM, Närhi TO, Syrjänen SM, Vallittu PK (2010) Bone response to prepolymerized versus in situ polymerized fiber reinforced composite—pilot study. J Dent Res 90:263–267
Vallittu PK, Ekstrand K (1999) In vitro cytotoxicity of fiber-polymethyl methacrylate composite used in dentures. J Oral Rehabil 26:666–671
Väkiparta M, Koskinen MK, Vallittu PK, Närhi TJ, Yli-Urpo A (2004) In vitro cytotoxicity of E-glass fiber weave preimpregnated with novel biopolymer. J Mater Sci Mater Mater Med 15:69–72
Tuusa S, Peltola M, Tirri T, Lassila LVJ, Vallittu PK (2007) Comparison of two glass fiber-reinforced composite structures as implant material in calvarial bone defect. Bioceram Key Eng Mater 361–363:471–474
Tuusa SM-R, Peltola MJ, Tirri T, Puska MA, Röyttä M, Aho H, Sandholm J, Lassila LVJ, Vallittu PK (2008) Reconstruction of critical size calvarial bone defect in rabbits with glass-fiber-reinforced composite with bioactive glass granule coating. J Biomed Mater Res B Appl Biomater 84:510–519
Ballo AM, Kokkari AK, Meretoja VV, Lassila LVJ, Vallittu PK, Närhi TO (2008) Osteoblast proliferation and maturation on bioactive fiber-reinforced composite. J Mater Sci Mater Mater Med 19(10):3169–3177
Ballo AM, Akca EA, Ozen T, Lassila LVJ, Vallittu PK, Närhi TO (2009) Bone tissue responses to glass fiber-reinforced composite implants—a histomorphometric study. Clin Oral Implants Res 20:608–615
Ballo AM, Cekic-Nagas I, Ergun G, Lassila L, Palmquist A, Thomsen P, Vallittu PK, Närhi TO (2014) Osseointegration of fiber-reinforced composite implants: a histological and ultrastructural observation. Dent Mater. doi:10.1016/j.dental.2014.08.361
Hench LL, West JK (1996) Biological applications of bioactive glasses. Life Chem Rep 13:187–241
Hench LL, Xynos ID, Polak JM (2004) Bioactive glasses for in situ tissue regeneration. J Biomater Sci Polym Ed 15(4):543–562
Välimäki VV, Aro HT (2006) Molecular basis for action of bioactive glasses as bone graft substitute. Scand J Surg 95(2):95–102
Boccaccini AR, Minay EJ, Krause D (2006) Bioglass coatings on superelastic NiTi wires by electrophoretic deposition (EPD). Electrophor Depos Fundam Appl II Key Eng Mater 314:219–224
Ojansivu M, Vanhatupa S, Björkvik L, Häkkänen H, Kellomäki M, Autio R, Ihalainen JA, Hupa L, Miettinen S (2015) Bioactive glass ions as strong enhancers of osteogenic differentiation in human adipose stem cells. Acta Biomater 21:190–203
Kinnunen I, Aitasalo K, Pöllönen M, Varpula M (2000) Reconstruction of orbital floor fractures using bioactive glass. J Craniomaxillofac Surg 28:229–234
Shinya A, Ballo AM, Lassila LV, Shinya A, Närhi TO, Vallittu PK (2011) Stress and strain analysis of the bone-implant interface: a comparison of fiber-reinforced composite and titanium implants utilizing 3-dimensional finite element study. J Oral Implantol 37:133–140
Lindfors NC, Hyvönen P, Nyyssönen M, Kirjavainen M, Kankare J, Gullichsen E, Salo J (2010) Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 47(2):212–218
Monfoulet LE, Becquart P, Marcaht D, Vandamme K, Bourguignon M, Pacard E, Viateau V, Petite H, Logeart-Avramoglu D (2014) The pH in the microenvironment of human mesenchymal stem cells is a critical factor for optimal osteogenesis in tissue-engineered constructs. Tissue Eng Part A 20:1827–1840
El-Gendy R, Kirkham J, Newby PJ, Mohanram Y, Boccaccini AR, Yang XB (2015) Investigating the vascularization of tissue-engineered bone constructs using dental pulp cells and 45S5 Bioglass scaffolds. Tissue Eng Part A 21:2034–2043
Zhang D, Leppäranta O, Munukka E, Ylänen H, Viljanen MK, Eerola E, Hupa M, Hupa L (2010) Antimicrobial effects and dissolution behaviour of six bioactive glasses. J Biomed Mater Res A 93:475–483
Leppäränta O, Vaahtio M, Peltola T, Zhang D, Hupa L, Hupa M, Ylänen H, Salonen JI, Viljanen MK, Eerola E (2008) Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J Mater Sci Mater Med 19:547–551
Munukka E, Leppäranta O, Korkeamäki M, Vaahtio M, Peltola T, Zhang D, Hupa L, Ylänen H, Salonen JI, Viljanen MK, Eerola E (2008) Bactericidal effects of bioactive glass on clinically important aerobic bacteria. J Mater Sci Mater Med 19:27–32
Lindfors NC, Heikkilä JT, Koski I, Mattila K, Aho AJ (2009) Bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J Biomed Mater Res B Appl Biomater 90(1):131–136
Lindfors NC, Koski I, Heikkilä JT, Mattila K, Aho AJ (2010) A prospective randomized 14-year follow-up study of bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J Biomed Mater Res B Appl Biomater 94(1):157–164
Peltola M, Vallittu PK, Vuorinen V, Aho A, Puntala A, Aitasalo K (2012) Novel composite implant in craniofacial bone reconstruction. Eur Arch Otorinolryngol 269:623–628
Piitulainen J, Posti JP, Aitasalo K, Vuorinen V, Vallittu P, Serlo W (2015) Pediatric cranial defect reconstruction using bioactive fiber reinforced composite implant: early outcomes. Acta Neurochir 157(4):681–687
Piitulainen JM, Kauko T, Aitasalo KMJ, Vuorinen V, Vallittu PK, Posti JP (2015) Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosug. doi:10.1016/j.wneu.2015.01.014
Shih YR, Hwang Y, Phadke A, Kang H, Hwang NS, Caro EJ, Nguyen S, Siu M, Theodorakis EA, Gianneschi NC, Veccio KS, Chien S, Lee OK, Varghese S (2014) Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signalling. Proc Natl Acad Sci 111:990–995
Nganga S, Moritz N, Kolakovic R, Jakobson K, Nyman JO, Travan A, Croserra M, Donati I, Vallittu PK, Sandler N (2014) Inkjet printing of Chitlac-nanosilver—a method to create bacteriostatic coatings for non-metallic bone implants. Biofabrication 6(4):041001. doi:10.1088/1758-5082/6/4/041001
Nganga S, Travan A, Marsish E, Donati I, Söderling E, Moritz N, Paoletti S, Vallittu PK (2013) In vitro antimicrobial behavior and biofilm inhibition of silver-polysaccharide on porous fibre reinforced composites for bone implants. J Mater Sci Mater Med 24:2775–2785
Baino F, Novajra G, Miguez-Pacheco V, Boccaccini AR, Vitale-Brovarone C (2016) Bioactive glasses: special applications outside the skeletal system. J Non-Cryst Solids 432:15–30
Baino F (2015) How can bioactive glasses be useful in ocular surgery? J Biomed Mater Res A 103:1259–1275
Acknowledgements
Research collaboration with the scientists of the FRC Research Group of the BioCity Turku Biomaterials and Medical Device Research Program (www.biomaterials.utu.fi) and with the Turku University Hospital and Oulu University Hospital are greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Author is inventor of the FRC implant system has a role as Member of the Board and shareholder in the company Skulle Implants Corporation.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vallittu, P.K. Bioactive glass-containing cranial implants: an overview. J Mater Sci 52, 8772–8784 (2017). https://doi.org/10.1007/s10853-017-0888-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-0888-x