Abstract
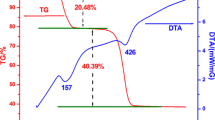
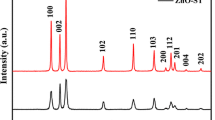
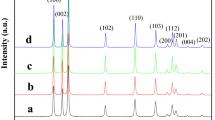
A simple facile method, i.e., thermal decarbonation of ZnCO3 hydroxides, was used to prepare a series of pure ZnO photocatalysts with controlled crystallite sizes, particle sizes, and morphologies. The ZnCO3 precursor was synthesized by direct wet carbonation in the presence of growth-control additives, i.e., organic solvents, surfactants, and low molecular weight polymers. The thermal decarbonation allows for producing ZnO photocatalysts with sizes and shapes varying from 80 ± 20 nm nonporous rhombohedral nanoparticles to 5 ± 0.5 µm porous particles, for a constant crystallite size of 64 ± 3 nm. The porous ZnO particles (5 ± 0.5 µm) exhibit two times larger photocatalytic activity for methanol oxidation than the nonporous ZnO nanoparticles (~180 ± 30 nm). The reasons for the higher photocatalytic activity are further investigated in this work. A possible mechanism for the formation of ZnCO3 hydroxides and their transformation into porous microsized ZnO particles and nonporous nanoparticles are carefully discussed.











Similar content being viewed by others
References
Zhang L, Wang W, Zhou L, Xu H (2007) Bi2WO6 nano- and microstructures: shape control and associated visible-light-driven photocatalytic activities. Small 3:1618–1625. doi:10.1002/smll.200700043
Lee HU, Lee SC, Lee Y-C et al (2014) Innovative three-dimensional (3D) eco-TiO2 photocatalysts for practical environmental and bio-medical applications. Sci Rep 4:6740. doi:10.1038/srep06740
McLaren A, Valdes-Solis T, Li G, Tsang SC (2009) Shape and size effects of ZnO nanocrystals on photocatalytic activity. J Am Chem Soc 131:12540–12541. doi:10.1021/ja9052703
Jang ES, Won J-H, Hwang S-J, Choy J-H (2006) Fine tuning of the face orientation of ZnO crystals to optimize their photocatalytic activity. Adv Mater 18:3309–3312. doi:10.1002/adma.200601455
Zhao T, Zhao Y, Jiang L (2013) Nano-/microstructure improved photocatalytic activities of semiconductors. Philos Trans A Math Phys Eng Sci 371:20120303. doi:10.1098/rsta.2012.0303
Becker J, Raghupathi KR, St. Pierre J et al (2011) Tuning of the crystallite and particle sizes of ZnO nanocrystalline materials in solvothermal synthesis and their photocatalytic activity for dye degradation. J Phys Chem C 115:13844–13850. doi:10.1021/jp2038653
Dodd AC, McKinley AJ, Saunders M, Tsuzuki T (2006) Effect of particle size on the photocatalytic activity of nanoparticulate zinc oxide. J Nanopart Res 8:43–51. doi:10.1007/s11051-005-5131-z
Manikandan E, Moodley MK, Sinha Ray S et al (2010) Zinc oxide epitaxial thin film deposited over carbon on various substrate by pulsed laser deposition technique. J Nanosci Nanotechnol 10:5602–5611
Park KT, Xia F, Kim SW et al (2013) Facile synthesis of ultrathin ZnO nanotubes with well-organized hexagonal nanowalls and sealed layouts: applications for lithium ion battery anodes. J Phys Chem C 117:1037–1043. doi:10.1021/jp310428r
Wang Y, Li X, Wang N et al (2008) Controllable synthesis of ZnO nanoflowers and their morphology-dependent photocatalytic activities. Sep Purif Technol 62:727–732. doi:10.1016/j.seppur.2008.03.035
Lao JY, Huang JY, Wang DZ, Ren ZF (2003) ZnO nanobridges and nanonails. Nano Lett 3:235–238. doi:10.1021/nl025884u
Zhao F, Zheng J-G, Yang X et al (2010) Complex ZnO nanotree arrays with tunable top, stem and branch structures. Nanoscale 2:1674. doi:10.1039/c0nr00076k
Kołodziejczak-Radzimska A, Jesionowski T (2014) Zinc oxide—from synthesis to application: a review. Materials (Basel) 7:2833–2881. doi:10.3390/ma7042833
Mahmud S, Abdullah M, Putrus G et al (2006) Nanostructure of ZnO fabricated via French process and its correlation to electrical properties of semiconducting varistors. Synth React Inorg Met-Org Nano-Met Chem 36:155–159
Moezzi A, McDonagh AM, Cortie MB (2012) Zinc oxide particles: synthesis, properties and applications. Chem Eng J 185–186:1–22. doi:10.1016/j.cej.2012.01.076
Clament Sagaya Selvam N, Vijaya JJ, Kennedy LJ (2012) Effects of morphology and Zr doping on structural, optical, and photocatalytic properties of ZnO nanostructures. Ind Eng Chem Res 51:16333–16345. doi:10.1021/ie3016945
Cho S, Jung S-H, Lee K-H (2008) Morphology-controlled growth of ZnO nanostructures using microwave irradiation: from basic to complex structures. J Phys Chem C 112:12769–12776. doi:10.1021/jp803783s
Wang J, Lee Y-J, Hsu JWP (2014) One-step synthesis of ZnO Nanocrystals in n-butanol with bandgap control: applications in hybrid and organic photovoltaic devices. J Phys Chem C 118:18417–18423. doi:10.1021/jp505058u
Zhang X, Qin J, Xue Y et al (2014) Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci Rep 4:4596. doi:10.1038/srep04596
McCune M, Zhang W, Deng Y (2012) High efficiency dye-sensitized solar cells based on three-dimensional multilayered ZnO nanowire arrays with “caterpillar-like” structure. Nano Lett 12:3656–3662. doi:10.1021/nl301407b
Duan X, Wang G, Wang H et al (2010) Orientable pore-size-distribution of ZnO nanostructures and their superior photocatalytic activity. CrystEngComm 12:2821. doi:10.1039/b922679f
Liu Y, Shi J, Peng Q, Li Y (2012) Self-assembly of ZnO nanocrystals into nanoporous pyramids: high selective adsorption and photocatalytic activity. J Mater Chem 22:6539. doi:10.1039/c2jm16729h
Xie H, Li Y, Jin S et al (2010) Facile fabrication of 3D-ordered macroporous nanocrystalline iron oxide films with highly efficient visible light induced photocatalytic activity. J Phys Chem C 114:9706–9712. doi:10.1021/jp102525y
Barhoum A, Ibrahim HM, Hassanein TF et al (2014) Preparation and characterization of ultra-hydrophobic calcium carbonate nanoparticles. IOP Conf Ser Mater Sci Eng 64:12037. doi:10.1088/1757-899X/64/1/012037
Nguyen T-D, Do T-O (2011) Nanocrystal. doi:10.5772/703
LaGrow AP, Ingham B, Toney MF, Tilley RD (2013) Effect of surfactant concentration and aggregation on the growth kinetics of nickel nanoparticles. J Phys Chem C 117:16709–16718. doi:10.1021/jp405314g
El-Sheikh SM, Barhoum A, El-Sherbiny S et al (2014) Preparation of superhydrophobic nanocalcite crystals using Box–Behnken design. Arab J Chem. doi:10.1016/j.arabjc.2014.11.003
Qi X, Balankura T, Zhou Y, Fichthorn KA (2015) How structure-directing agents control nanocrystal shape: polyvinylpyrrolidone-mediated growth of Ag nanocubes. Nano Lett 15:7711–7717. doi:10.1021/acs.nanolett.5b04204
Bullen CR, Mulvaney P (2004) Nucleation and growth kinetics of CdSe nanocrystals in octadecene. Nano Lett 4:2303–2307. doi:10.1021/nl0496724
Barhoum A, Rehan MF, Rahier H et al (2016) Seed-mediated hot injection synthesis of tiny Ag nanocrystals on nanoscale solid supports and reaction mechanism. ACS Appl Mater Interfaces. doi:10.1021/acsami.5b10405
van Embden J, Mulvaney P (2005) Nucleation and growth of CdSe nanocrystals in a binary ligand system. Langmuir 21:10226–10233. doi:10.1021/la051081l
Potti PR, Srivastava VC (2012) Comparative studies on structural, optical, and textural properties of combustion derived ZnO prepared using various fuels and their photocatalytic activity. Ind Eng Chem Res 51:7948–7956. doi:10.1021/ie300478y
Holzwarth U, Gibson N (2011) The Scherrer equation versus the “Debye–Scherrer equation. Nat Nanotechnol. doi:10.1038/nnano.2011.145
Christy AA, Kvalheim OM, Velapoldi RA (1995) Quantitative analysis in diffuse reflectance spectrometry: a modified Kubelka–Munk equation. Vib Spectrosc 9:19–27. doi:10.1016/0924-2031(94)00065-O
Nash T (1953) The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J 55:416–421
Fateh R, Dillert R, Bahnemann D (2014) Self-cleaning properties, mechanical stability, and adhesion strength of transparent photocatalytic TiO(2)–ZnO coatings on polycarbonate. ACS Appl Mater Interfaces 6:2270–2278. doi:10.1021/am4051876
Moghaddam J, Ghaffari SB, Sarraf-Mamoory R, Mollaesmail S (2014) The study on the crystallization conditions of Zn5(OH)6(CO3)2 and its effect on precipitation of ZnO nanoparticles from purified zinc ammoniacal solution. Synth React Inorg Met-Org Nano-Met Chem 44:895–901. doi:10.1080/15533174.2012.740738
Kanari N, Mishra D, Gaballah I, Dupré B (2004) Thermal decomposition of zinc carbonate hydroxide. Thermochim Acta 410:93–100. doi:10.1016/S0040-6031(03)00396-4
El-Sheikh SM, El-Sherbiny S, Barhoum A, Deng Y (2013) Effects of cationic surfactant during the precipitation of calcium carbonate nano-particles on their size, morphology, and other characteristics. Coll Surf A Physicochem Eng Asp 422:44–49. doi:10.1016/j.colsurfa.2013.01.020
Barhoum A, Van Lokeren L, Rahier H et al (2015) Roles of in situ surface modification in controlling the growth and crystallization of CaCO3 nanoparticles, and their dispersion in polymeric materials. J Mater Sci 50:7908–7918. doi:10.1007/s10853-015-9327-z
Barhoum A, Rahier H, Abou-Zaied RE et al (2014) Effect of cationic and anionic surfactants on the application of calcium carbonate nanoparticles in paper coating. ACS Appl Mater Interfaces 6:2734–2744. doi:10.1021/am405278j
Barhoum A, Van Assche G, Makhlouf ASH et al (2015) A green, simple chemical route for the synthesis of pure nanocalcite crystals. Cryst Growth Des 15:573–580. doi:10.1021/cg501121t
Kumar SG, Rao KSRK (2015) Zinc oxide based photocatalysis: tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv 5:3306–3351. doi:10.1039/C4RA13299H
Gebauer D, Völkel A, Cölfen H (2008) Stable prenucleation calcium carbonate clusters. Science 322:1819–1822. doi:10.1126/science.1164271
Song R-Q, Cölfen H (2011) Additive controlled crystallization. CrystEngComm 13:1249. doi:10.1039/c0ce00419g
Peng Y, Wang F, Wang Z et al (2015) Two-step nucleation mechanism in solid–solid phase transitions. Nat Mater 14:101–108. doi:10.1038/nmat4083
Kawasaki T, Tanaka H (2010) Formation of a crystal nucleus from liquid. Proc Natl Acad Sci U S A 107:14036–14041. doi:10.1073/pnas.1001040107
Sun Y, Wang L, Yu X, Chen K (2012) Facile synthesis of flower-like 3D ZnO superstructures via solution route. CrystEngComm 14:3199. doi:10.1039/c2ce06335b
Panmand RP, Sethi YA, Kadam SR et al (2015) Self-assembled hierarchical nanostructures of Bi2WO6 for hydrogen production and dye degradation under solar light. CrystEngComm 17:107–115. doi:10.1039/C4CE01968G
Shi W, Huo L, Wang H et al (2006) Hydrothermal growth and gas sensing property of flower-shaped SnS2 nanostructures. Nanotechnology 17:2918–2924. doi:10.1088/0957-4484/17/12/016
Thanh NTK, Maclean N, Mahiddine S (2014) Mechanisms of nucleation and growth of nanoparticles in solution. Chem Rev 114:7610–7630. doi:10.1021/cr400544s
Wang F, Wang X (2014) Mechanisms in the solution growth of free-standing two-dimensional inorganic nanomaterials. Nanoscale 6:6398–6414. doi:10.1039/c4nr00973h
Jradi K, Maury C, Daneault C (2015) Contribution of TEMPO-oxidized cellulose gel in the formation of flower-like zinc oxide superstructures: characterization of the TOCgel/ZnO composite films. Appl Sci 5:1164–1183. doi:10.3390/app5041164
Hong L, Li Q, Lin H, Li Y (2009) Synthesis of flower-like silver nanoarchitectures at room temperature. Mater Res Bull 44:1201–1204. doi:10.1016/j.materresbull.2009.01.017
Bhattacharyya L, Rohrer JS (2012) Applications of ion chromatography for pharmaceutical and biological products. Wiley, Hoboken
Vos JG, Forster RJ, Keyes TE (2003) Interfacial supramolecular assemblies. Wiley, Chichester
Wang Y, Jiang Z-H, Yang F-J (2006) Preparation and photocatalytic activity of mesoporous TiO2 derived from hydrolysis condensation with TX-100 as template. Mater Sci Eng B 128:229–233. doi:10.1016/j.mseb.2005.12.004
Moezzi A, Cortie M, Dowd A, McDonagh A (2014) On the formation of nanocrystalline active zinc oxide from zinc hydroxide carbonate. J Nanoparticle Res 16:2344. doi:10.1007/s11051-014-2344-z
Dollimore D, France JA, Krupay BW, Whitehead R (1980) Kinetic aspects of the thermal decomposition of zinc carbonate. Thermochim Acta 36:343–349. doi:10.1016/0040-6031(80)87029-8
Yang L, Liu Z (2007) Study on light intensity in the process of photocatalytic degradation of indoor gaseous formaldehyde for saving energy. Energy Convers Manag 48:882–889. doi:10.1016/j.enconman.2006.08.023
Wang C, Rabani J, Bahnemann DW, Dohrmann JK (2002) Photonic efficiency and quantum yield of formaldehyde formation from methanol in the presence of various TiO2 photocatalysts. J Photochem Photobiol A Chem 148:169–176. doi:10.1016/S1010-6030(02)00087-4
Park Y, Kim W, Monllor-Satoca D et al (2013) Role of interparticle charge transfers in agglomerated photocatalyst nanoparticles: demonstration in aqueous suspension of dye-sensitized TiO2. J Phys Chem Lett 4:189–194. doi:10.1021/jz301881d
Sun Y-F, Liu S-B, Meng F-L et al (2012) Metal oxide nanostructures and their gas sensing properties: a review. Sensors (Basel) 12:2610–2631. doi:10.3390/s120302610
Schneider J, Matsuoka M, Takeuchi M et al (2014) Understanding TiO2 photocatalysis: mechanisms and materials. Chem Rev 114:140919080959008. doi:10.1021/cr5001892
Hagfeldt A, Graetzel M (1995) Light-induced redox reactions in nanocrystalline systems. Chem Rev 95:49–68. doi:10.1021/cr00033a003
Acknowledgements
This work was performed during research stay of Dr. Ahmed Barhoum at Institute of Technical Chemistry, Leibniz Universität Hannover and Institut Européen des Membranes, Université Montpellier, France. The authors would like to thank the FWO-Research Foundation Flanders (Grant No V450315N and V423116N), Strategic Initiative Materials in Flanders (SBO- Project No. 130529 - Insitu), European Regional Development Fund (Nanokomp as part of the program “Europa fördert Niedersachsen”; Grant No. WA3-80125215), and ERLUS AG for financial support. Note that the authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Barhoum, A., Melcher, J., Van Assche, G. et al. Synthesis, growth mechanism, and photocatalytic activity of Zinc oxide nanostructures: porous microparticles versus nonporous nanoparticles. J Mater Sci 52, 2746–2762 (2017). https://doi.org/10.1007/s10853-016-0567-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0567-3