Abstract
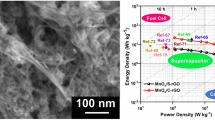
A facile method was developed to prepare MnO2/holey graphene oxide (MnO2/HGO) materials based on graphene oxide (GO) flakes for supercapacitor applications. FESEM images show that MnO2 nanorods were formed on the surface of HGO flakes, serving as spacers and preventing the HGO layers from stacking. This provides pathways between the layers for the electrolyte to access the bulk active materials. By introducing the high intrinsic capacitance MnO2 nanorods together with the modified 3-D structure, capacitance increases to 71.0 F/g compared with 30.0 F/g of GO. More pathways were created by nitric acid etching holes on the surface of the GO. This 3-D holey MnO2/HGO structure achieves a capacitance of 117.45 F/g, which is 1.65 times higher than that of MnO2/GO composite and 3.9 times higher than that of GO only. BET surface area, XRD, and AC impedance were also used to analyze the possible reasons for the enhanced electrochemical performance.









Similar content being viewed by others
References
Markoulidisa F, Leia C, Lekakoua C, Duffb D, Khalilb S, Martoranac B, Cannavaroc I (2014) A method to increase the energy density of supercapacitor cells by the addition of multiwall carbon nanotubes into activated carbon electrodes. Carbon 68:58–66
Mateyshina Y, Ulihin A, Samarov A, Barnakov C, Uvarov N (2013) Nanoporous carbon-based electrode materials for supercapacitors. Solid State Ion 251:59–61
Mezavilla S, Zanella C, Aravind PuR, Yolpe CD, Soraru GD (2012) Carbon xerogels as electrodes for supercapacitors. The influence of the catalyst concentration on the microstructure and on the electrochemical properties. J Mater Sci 47:7175–7180. doi:10.1007/s10853-012-6662-1
Tao J, Liu N, Ma W, Ding L, Li L, Su J, Gao Y (2013) Solid-state high performance flexible supercapacitors based on polypyrrole-MnO2-carbon fiber hybrid structure. Sci Rep 3:2286. doi:10.1038/srep02286
Wang Y, Shi Z, Huang Y, Ma Y, Wang C, Chen M, Chen Y (2009) Supercapacitor devices based on graphene materials. J Phys Chem C 113:13103–13107
Yao W, Wang J, Li H, Lu Y (2014) Flexible α-MnO2 paper formed by millimeter-long nanowires for supercapacitor electrodes. J Power Sources 247:824–830. doi:10.1016/j.jpowsour.2013.09.039
Hu J, Kang Z, Li F, Huang X (2014) Graphene with three-dimensional architecture for high performance supercapacitor. Carbon 67:221–229. doi:10.1016/j.carbon.2013.09.085
Kim Y-S, Kumar K, Fisher FT, Yang E-H (2012) Out-of-plane growth of CNTs on graphene for supercapacitor applications. Nanotechnology 23:015301–015308. doi:10.1088/0957-4484/23/1/015301
Jiang H, Lee PS, Li C (2013) 3D carbon based nanostructures for advanced supercapacitors. Energy Environ Sci 6:41–53. doi:10.1039/c2ee23284g
Conte M (2010) Supercapacitors technical requirements for new applications. Fuel Cells 10(5):806–818
Sopcic S, Rokovic MK, Mandic Z, Róka A, Inzelt G (2011) Mass changes accompanying the pseudocapacitance of hydrous RuO2 under different experimental conditions. Electrochim Acta 56:3543–3548. doi:10.1016/j.electacta.2010.10.035
Li Z-S, Wang H-Q, Huang Y-G, Li Q-Y, Wang X-Y (2010) Manganese dioxide-coated activated mesocarbon microbeads for supercapacitors in organic electrolyte. Colloids Surf A 366:104–109. doi:10.1016/j.colsurfa.2010.05.031
Su L, Gong L, Lü H, Xü Q (2014) Enhanced low-temperature capacitance of MnO2 nanorods in a redox-active electrolyte. J Power Sources 248:212. doi:10.1016/j.jpowsour.2013.09.047
Yang J, Lan T, Liu J, Song Y, Wei M (2013) Supercapacitor electrode of hollow spherical V2O5 with a high pseudocapacitance in aqueous solution. Electrochim Acta 105:489–495. doi:10.1016/j.electacta.2013.05.023
Nakayama M, Tanaka A, Sato Y, Tonosaki T, Ogura K (2005) Electrodeposition of manganese and molybdenum mixed oxide thin films and their charge storage properties. Langmuir 21:5907–5913
Dubal DP, Dhawale DS, Salunkhe RR, Lokhande CD (2010) A novel chemical synthesis of Mn3O4 thin film and its stepwise conversion into birnessite MnO2 during super capacitive studies. J Electroanal Chem 647:60–65
He Y, Chen W, Li X, Zhang Z, Fu J, Zhao C, Xie E (2013) Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 7:174–182. doi:10.1021/nn304833s
Liu Y, He D, Wu H, Duan J, Zhang Y (2015) Hydrothermal self-assembly of manganese dioxide/manganese carbonate/reduced graphene oxide aerogel for asymmetric supercapacitors. Electrochim Acta 164:154–162
Huang Y, Zhu M, Meng W, Fu Y, Wang Z, Huang Y, Pei Z, Zhi C (2015) Robust reduced graphene-oxide paper fabricated by household non-stick frying pan: large-area freestanding flexible substrate for supercapacitor. RSC Adv 5:33981–33989. doi:10.1039/c5ra02868j
Wang L, Deng D, Ng KYS (2013) Facile one-step synthesis of MnO2 nanowires on graphene under mild conditions for application in supercapacitors. J Mater Sci 48(18):6410–6417. doi:10.1007/s10853-013-7441-3
Zhao X, Hayner CM, Kung MC, Kung HH (2011) Flexible holey graphene paper electrodes with enhanced rate capability for energy storage applications. ACS Nano 5(11):8739–8749
Lee JK, Smith KB, Hayner CM, Kung HH (2010) Silicon nanoparticles–graphene paper composites for Li ion battery anodes. Chem Commun 46:2025–2027
Balakrishnan A, Subramanian KRV (2014) Nanostructured ceramic oxides for supercapacitor applications. CRC Press, New York
Gamby J, Taberna PL, Simon P, Fauvarque JF, Chesneau M (2001) Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J Power Sources 101:109–116
Zhang L, Zhang F, Yang X, Long G, Wu Y, Zhang T, Leng K, Huang Y, Ma Y, Yu A, Chen Y (2013) Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci Rep 3:1408–1416
Wang Y, Guo CX, Liu J, Chen T, Yang H, Li CM (2011) CeO2 nanoparticles/graphene nanocomposite-based high performance supercapacitor. Dalton Trans 40:6388–6391
Choi BG, Yang M, Hong WH, Choi JW, Huh YS (2012) 3D macroporous graphene frameworks for supercapacitors with high energy and power densities. ACS Nano 6(5):4020–4028. doi:10.1021/nn3003345
Acknowledgement
Financial support from the Department of Energy (Grant DEFG36-05GO85005) for this research is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Deng, D., Salley, S.O. et al. Facile synthesis of 3-D composites of MnO2 nanorods and holey graphene oxide for supercapacitors. J Mater Sci 50, 6313–6320 (2015). https://doi.org/10.1007/s10853-015-9169-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9169-8