Abstract
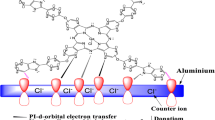
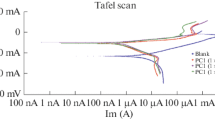
The corrosion inhibition characteristics of quaternized 1,(4)-tetrakis[(2-mercapto)pyridine]phthalocyanine (I) and 2,3-octakis[(2-mercapto)pyridine] phthalocyanine (II) on aluminum in 0.1 M HCl solution has been studied by means of potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) techniques. The maximum inhibition efficiency was obtained for compound I with two-electrochemical techniques applied. Langmuir isotherm fits well the experimental data. The inhibition efficiency increases with increase in the phthalocyanine concentration, but decreases with an increase in temperature. The phthalocyanines act predominately as cathodic inhibitor.










Similar content being viewed by others
References
Umoren S, Obot I, Obi-Egbedi N (2009) J Mater Sci 44:274. doi:10.1007/s10853-008-3045-8
Aghion E, Lulu N (2009) J Mater Sci 44:4279. doi:10.1007/s10853-009-3634-1
Metikos-Hukovic M, Babic R, Grubac Z (2002) J Appl Electrochem 32:35
Babic-Samardzija K, Hackerman N, Sovilj SP, Jovanovic VM (2008) J Solid State Electrochem
Yurt A, Ulutas S, Dal H (2006) Appl Surf Sci 253:919
Song M, Chen KH (2008) J Mater Sci 43:5265. doi:10.1007/s10853-008-2573-6
Wang CY, Wu GH, Zhang Q et al (2008) J Mater Sci 43:3327. doi:10.1007/s10853-008-2506-4
Xu WL, Yue TM, Man HC (2008) J Mater Sci 43:942. doi:10.1007/s10853-007-2208-3
Pyun S, Moon S-M (2000) J Solid State Electrochem 4:267
Branzoi V, Golgovici F, Branzoi F (2002) Mater Chem Phys 78:122
Ashassi-Sorkhabi H, Shabani B, Aligholipour B, Seifzadeh D (2006) Appl Surf Sci 252:4039
Aoki IV, Guedes IC, Maranhao SLA (2002) J Appl Electrochem 32:915
Zhao P, Liang Q, Li Y (2005) Appl Surf Sci 252:1596
Agarwala VS (1984) Proc Int Cong Metal Corros 1:380
George RD, Snow AW (1995) J Heterocycl Chem 32:495
Young JG, Onyebuagu W (1990) J Org Chem 55:2155
Sehlotho N, Durmuş M, Ahsen V, Nyokong T (2008) Inorg Chem Commun 11:479
Smith TD, Livorness J, Taylor HJ (1983) Chem Soc Dalton Trans 1391–1400
Sheldon RA (ed) (1994) Metalloporphyrins in catalytic oxidation. Dekker, New York
Duke SO, Reberz CA (eds) (1994) Porphyric pesticides—chemistry, toxicology, and pharmaceutical applications. American Chemical Society, Washington, DC
Tang CW (1986) Appl Phys Lett 48:183
Philip R, Ravikanth M, Kumar GR (1999) Opt Commun 165:91
Manas ES, Spano FC, Chen LX (1997) J Chem Phys 107(3):707
Abd el Rehim SS, Hassan HH, Amin MA (2001) Mater Chem Phys 70:64
Lee EJ, Pyun SI (1995) Corros Sci 37:157
Brett CMA (1992) Corros Sci 33:203
Brett CMA (1990) J Appl Electrochem 20:1000
Gojic M, Horvat R, Metikos-Hukovic M (1990) In: Proceedings of 6th European symposium corrosion inhibitors, vol 2, Ferrara, Italy, p 1099
Avci G (2008) Colloids Surf A Physicochem Eng Aspects 317:730
Donahue FM, Nobe K (1965) J Electrochem Soc 112:677
Khamis E, Bellucci F, Latanision RM, El-Ashry ESH (1991) Corrosion 47:677
Saleh MM (2006) Mater Chem Phys 98:83
Acknowledgement
Thanks to author Devrim Atilla and Mahmut Durmuş for synthesizing phthalocyanines. This project was supported by TÜBITAK (project no. MAG107M476).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özdemir, O.K., Aytaç, A., Atilla, D. et al. Corrosion inhibition of aluminum by novel phthalocyanines in hydrochloric acid solution. J Mater Sci 46, 752–758 (2011). https://doi.org/10.1007/s10853-010-4808-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4808-6