Abstract
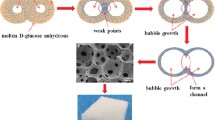
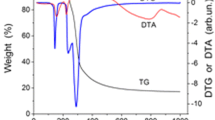
A novel process for preparation low density carbon foams from sucrose has been demonstrated. A resin prepared by heating aqueous acidic sucrose solution when heated in an open Teflon mould at 120 °C undergoes foaming and then setting in to a solid organic foam. The solid organic foam undergoes carbonization in air by dehydration at 250 °C under isothermal condition. Carbon foams thus obtained sintered at temperature in the range 600–1,400 °C showed density in the range 115–145 mg/cc and electrical conductivity in the range 1.5 × 10−5 to 0.2 ohm−1 cm−1, respectively. The carbon foams contain spherical cells of size in the range 450–850 μm and the cells are interconnected through circular or oval shape windows of size in the range 80–300 μm. The carbon foam samples sintered at 1,400 °C showed compressive strength of 0.89 MPa.














Similar content being viewed by others
References
Cowlard FC, Lewis JC (1967) J Mater Sci 2:507
Knippenberg WF, Dersmacher B (1976) Philips Tech Rev 36:93
Noda T, Inagaki M, Yamada S (1969) J Non-Cryst Solids 1:93
Klett RD (1975) High temperature insulating carbonaceous materials. US Patent 3,914,392
Bonzom A, Crepaux AP, Montard AMEJ (1981) Process for preparing pitch foams and products so produced. US Patent, 4,276,246
Marek R, Udichak W (1975) Foam carbonization and resulting structure. US Patent 3,922,334
Vinton C, Franklin C (1975) Method for the preparation of carbon structure. US Patent 3,927,186
Spradling DM, Andrew Guth R (2003) Adv Mater Process 11:29
Klett JW, Mcmillan AD, Gallego NC, Walls CA (2004) J Mater Sci 39:3659
Raley CF Jr, Asher DR (1976) Process for preparing macroporous open cell carbon foam from normally crystalline vinylidene chloride polymer. US Patent 3,960,770
Droege MW (1999) Low density open cell organic foam, low density carbon foams, and method for preparing the same. US Patent 5,945,084
Stankiewicz EP (2000) Method for producing controlled aspect ratio reticulated carbon foam and the resultant foam. US Patent 6,103,149
Vinton CS, Franklin CH (1979) Activated reticulated or unreticulated carbon structures. US Patent 4,154,704
Pekala RW (1989) Low density, resorcinol – formaldehyde aerogels. US Patent 4,873,218
Mukai SR, Tamitsuji C, Nishihara H, Tamon H (2005) Carbon 43:2628
Nishihara H, Mukai SR, Tamon H (2004) Carbon 42:899
Inagaki M, Morishita T, Kuno A, Kito T, Hirano M, Suwa T, Kusakawa K (2004) Carbon 42:497
Yamashita J, Ojima T, Shioya M, Hatori H, Yamada Y (2003) Carbon 41:285
Klett RD (1975) High temperature insulating carbonaceous material. US Patent 3,914,392
Nagle DC, Byrne CE (2003) Carbonized wood and materials formed therefrom. US Patent 6,670,039
Stiller AH, Plucinski J, Yocum A (2003) Method of making carbon foam. US Patent 6,544,491
Fujii M, Minohata M (1990) Method for producing elastic graphite structures. US Patent 4,908,200
Hardcastle LA, Sheppard RG, Dingus DF (2003) Process for making carbon foam induced by process depressurization. US Patent 6,576,168
Klett JW (2003) Pitch-based carbon foam and composites. US Patent 6,663,842
Yamada Y, Imamura T, Honda H, Fujii M, Minohata M (1989) Graphite structures and method for production thereof. US Patent 4,873,071
Li TQ, Wang CY, An BX, Wang H (2005) Carbon 43:2030
Klett JW, Mcmillan AD, Gallego NC, Burchell TD, Walls CA (2004) Carbon 42:1849
Bohme K, Einicke WD, Klepel O (2005) Carbon 43:1918
Finar IL (1973) Organic chemistry volume. I. The fundamental principles. Longman, London, pp 503–530
Kinoshita K (1988) Carbon electrochemical and physical properties. Wiley & Sons, USA, pp 22–75
Biscoe J, Warren BE (1942) J Appl Phys 13:364
Jenkins GM, Kawamura K (1976) Polymeric carbons, carbon fibers, glass and char. Cambridge University press, New York
Long JC (1978) In: Grayson M (ed) Encyclopedia of chemical technology, vol 4. John Wiley and Sons, New York, pp 556–560
Acknowledgements
The authors thank Dr. J. Narayana Das, Director, Naval Materials Research Laboratory for his support and keen interest in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prabhakaran, K., Singh, P.K., Gokhale, N.M. et al. Processing of sucrose to low density carbon foams. J Mater Sci 42, 3894–3900 (2007). https://doi.org/10.1007/s10853-006-0481-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-0481-1