Abstract
Aim/objective
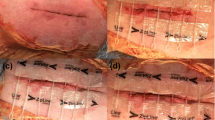
Aim to evaluate the safety and effectiveness of a pocket compression device in patients undergoing cardiovascular implantable electronic device (CIED) implantation.
Material and methods
This randomized controlled trial included 54 patients in the control group (elastic adhesive tape) and 54 patients in the experimental group (pocket compression device). A sandbag was positioned over the pocket after CIED implantation, using elastic adhesive tape in the control group and a novel pocket compression device in the experimental group. The primary outcome was the incidence of pocket hematomas, and the secondary outcomes were adverse skin reactions, the need for sandbag positional adjustments, and patient comfort.
Results
The distributions of age, sex, and clinical and procedure characteristics was not significantly different between the two groups (P>0.05). The occurrence of hematomas was lower when using the pocket compression device than in the control group (13.0% vs. 44.4%, P<0.001). The incidence rates of hematomas of grades 1 and the grade 2 hematomas were significantly lower in the experimental group than in the control group (13.0% vs. 31.5%, 0% vs. 11.1% respectively, [all P<0.05]). The incidence rates of adverse skin reactions and sandbag positional adjustments were much lower and the patient comfort score was significantly higher in the experimental group than in the control group (all P<0.001).
Conclusion
A pocket compression device can significantly decrease the incidence of pocket hematomas, adverse skin reactions, and positional adjustments when patients are undergoing CIED, which can improve patient comfort and decrease the nursing workload.
ClinicalTrials.gov
NCT04389398




Similar content being viewed by others
References
Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA 3rd, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2012;144(6):e127–45.
Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34(29):2281–329.
Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, Carrillo R, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14(12):e503–51.
Yang X, Wang Z, Zhang Y, Yin X, Hou Y. The safety and efficacy of antithrombotic therapy in patients undergoing cardiac rhythm device implantation: a meta-analysis. Europace. 2015;17(7):1076–84.
Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu B, et al. Clinically significant pocket hematoma increases long-term risk of device infection: BRUISE CONTROL INFECTION study. J Am Coll Cardiol. 2016;67(11):1300–8.
Reynolds MR, Cohen DJ, Kugelmass AD, Brown PP, Becker ER, Culler SD, et al. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47(12):2493–7.
Baddour LM, Epstein AE, Erickson CC, Knight BP, Levison ME, Lockhart PB, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121(3):458–77.
Schaller RD. A comparison of two insulated electrocautery blades: what is the thermal damage effect on transvenous cardiac device leads? J Innov Card Rhythm Manag. 2018;9(12):3436–8.
Piromchai P, Vatanasapt P, Reechaipichitkul W, Phuttharak W, Thanaviratananich S. Is the routine pressure dressing after thyroidectomy necessary? A prospective randomized controlled study. BMC Ear Nose Throat Disord. 2008;8:1.
Hu J. Wound compression fixing device after pacemaker implantation. China National Intellectual Property Administration, Editor. 2016: China.
Des F, Miracapillo G, Cresti A, Severi S, Airaksinen KE. Pocket hematoma: a call for definition. Pacing Clin Electrophysiol. 2015;38(8):909–13.
Lattuca B, Barber-Chamoux N, Alos B, Sfaxi A, Mulliez A, Miton N, et al. Impact of video on the understanding and satisfaction of patients receiving informed consent before elective inpatient coronary angiography: a randomized trial. Am Heart J. 2018;200:67–74.
Larsson P, John MT, Nilner K, List T. Reliability and validity of the Orofacial Esthetic Scale in prosthodontic patients. Int J Prosthodont. 2010;23(3):257–62.
Koh Y, Bingham NE, Law N, Le D, Mariani JA. Cardiac implantable electronic device hematomas: risk factors and effect of prophylactic pressure bandaging. Pacing Clin Electrophysiol. 2017;40(7):857–67.
Henley J, Brewer JD. Newer hemostatic agents used in the practice of dermatologic surgery. Dermatol Res Pract. 2013;2013:279289.
Kiuchi K, Okajima K, Tanaka N, Yamamoto Y, Sakai N, Kanda G, et al. Novel compression tool to prevent hematomas and skin erosions after device implantation. Circ J. 2015;79(8):1727–32.
Turagam MK, Nagarajan DV, Bartus K, Makkar A, Swarup V. Use of a pocket compression device for the prevention and treatment of pocket hematoma after pacemaker and defibrillator implantation (STOP-HEMATOMA-I). J Interv Card Electrophysiol. 2017;49(2):197–204.
Awada H, Geller JC, Brunelli M, Ohlow MA. Pocket related complications following cardiac electronic device implantation in patients receiving anticoagulation and/or dual antiplatelet therapy: prospective evaluation of different preventive strategies. J Interv Card Electrophysiol. 2019;54(3):247–55.
Demir GG, Guler GB, Guler E, Gunes H, Kizilirmak F, Karaca IO, et al. Pocket haematoma after cardiac electronic device implantation in patients receiving antiplatelet and anticoagulant treatment: a single-centre experience. Acta Cardiol. 2017;72(1):47–52.
Ferretto S, Mattesi G, Migliore F, Susana A, De Lazzari M, Iliceto S, et al. Clinical predictors of pocket hematoma after cardiac device implantation and replacement. J Cardiovasc Med (Hagerstown). 2020;21(2):123–7.
Dai Y, Chen KP, Hua W, Zhang JT, Zhang S. Dual antiplatelet therapy increases pocket hematoma complications in Chinese patients with pacemaker implantation. J Geriatr Cardiol. 2015;12(4):383–7.
Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368(22):2084–93.
Acknowledgements
The authors would like to acknowledge all the staff at unit 1 of Department of Cardiology, the First Affiliated Hospital of Xi’an Jiaotong University for their tireless work and professionalism.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, J., Zheng, J., Liu, X. et al. Effect of a pocket compression device on hematomas, skin reactions, and comfort in patients receiving a cardiovascular implantable electronic device: a randomized controlled trial. J Interv Card Electrophysiol 63, 275–281 (2022). https://doi.org/10.1007/s10840-021-00973-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-021-00973-5