Abstract
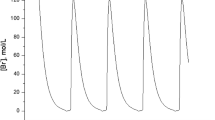
Intensive computational and theoretical work has led to the development of multiple mathematical models for bursting in respiratory neurons in the pre-Bötzinger Complex (pre-BötC) of the mammalian brainstem. Nonetheless, these previous models have not captured the pre-inspiratory ramping aspects of these neurons’ activity patterns, in which relatively slow tonic spiking gradually progresses to faster spiking and a full-blown burst, with a corresponding gradual development of an underlying plateau potential. In this work, we show that the incorporation of the dynamics of the extracellular potassium ion concentration into an existing model for pre-BötC neuron bursting, along with some parameter adjustments, suffices to induce this ramping behavior. Using fast-slow decomposition, we show that this activity can be considered as a form of parabolic bursting, but with burst termination at a homoclinic bifurcation rather than as a SNIC bifurcation. We also investigate the parameter-dependence of these solutions and show that the proposed model yields a greater dynamic range of burst frequencies, durations, and duty cycles than those produced by other models in the literature.







Similar content being viewed by others
Data availability
Not applicable.
Code availibility
The XPP and MATLAB codes used in this work will be uploaded to ModelDB, where they will be freely available, upon acceptance of this work for publication.
References
Amzica, F., Massimini, M., & Manfridi, A. (2002). Spatial buffering during slow and paroxysmal sleep oscillations in cortical networks of glial cells in vivo. Journal of Neuroscience, 22(3), 1042–1053. https://doi.org/10.1523/JNEUROSCI.22-03-01042.2002
Bacak, B. J., Kim, T., Smith, J. C., Rubin, J. E., & Rybak, I. A. (2016a). Mixed-mode oscillations and population bursting in the pre-Bötzinger complex. eLife 5:e13403. https://doi.org/10.7554/eLife.13403
Bacak, B. J., Segaran, J., & Molkov, Y. (2016b). Modeling the effects of extracellular potassium on bursting properties in pre-Bötzinger complex neurons. Journal of Computational Neuroscience, 40(2), 231–245. https://doi.org/10.1007/s10827-016-0594-8
Barreto, E., & Cressman J. R. (2011). Ion concentration dynamics as a mechanism for neuronal bursting. Journal of Biological Physics, 37(3), 361–373. https://doi.org/10.1007/s10867-010-9212-6
Baylor, D. A., & Nicholls, J. G. (1969). Changes in extracellular potassium concentration roduced by neuronal activity in the central nervous system of the leech. Journal of Physiology, 203(3), 555–569. https://doi.org/10.1016/0166-2236(96)10040-0
Beckner, M. E. (2020). A roadmap for potassium buffering/dispersion via the glial network of the CNS. Neurochemistry international, 136, 104727. https://doi.org/10.1016/j.neuint.2020.104727
Ben-Tal, A., & Smith, J. C. (2008). A model for control of breathing in mammals: coupling neural dynamics to peripheral gas exchange and transport. Journal of Theoretical Biology, 251(3), 480–497. https://doi.org/10.1016/10.1016/j.jtbi.2007.12.018
Bertram, R., & Rubin, J. E. (2017). Multi-timescale systems and fast-slow analysis. Mathematical Biosciences, 287, 105–121. https://doi.org/10.1016/j.mbs.2016.07.003
Butera, R. J., Rinzel, J., & Smith, J. C. (1999). Models of respiratory rhythm generation in the pre-Bötzinger complex. I. Bursting pacemaker neurons. Journal of Neurophysiology, 82(1), 382–397. https://doi.org/10.1152/jn.1999.82.1.382
Chevalier, M., Toporikova, N., Simmers, J., & Thoby-Brisson, M. (2016). Development of pacemaker properties and rhythmogenic mechanisms in the mouse embryonic respiratory network. elife 5:e16125. https://doi.org/10.7554/eLife.16125
Clausen, M. V., Hilbers, F., & Poulsen, H. (2017). The structure and function of the Na, K-ATPase isoforms in health and disease. Frontiers in Physiology, 8, 371. https://doi.org/10.3389/fphys.2017.00371
Currin, C. B., Trevelyan, A. J., Akerman, C. J., & Raimondo, J. V. (2020). Chloride dynamics alter the input-output properties of neurons. PLoS Computational Biology, 16(5), e1007932. https://doi.org/10.1371/journal.pcbi.1007932
Del Negro, C. A., Johnson, S. M., Butera, R. J., & Smith, J. C. (2001). Models of respiratory rhythm generation in the pre-Bötzinger complex. III. Experimental tests of model predictions. Journal of Neurophysiology, 86(1), 59–74. https://doi.org/10.1152/jn.2001.86.1.59
Del Negro, C. A., Koshiya, N., Butera, R. J., & Smith, J. C. (2002). Persistent sodium current, membrane properties and bursting behavior of pre-Bötzinger complex inspiratory neurons in vitro. Journal of Neurophysiology, 88(5), 2242–2250. https://doi.org/10.1152/jn.00081.2002
Del Negro, C. A., Morgado-Valle, C., Hayes, J. A., Mackay, D. D., Pace, R. W., Crowder, E. A., & Feldman, J. L. (2005). Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. Journal of Neuroscience, 25(2), 446–453. https://doi.org/10.1523/JNEUROSCI.2237-04.2005
Del Negro, C. A., Funk, G. D., & Feldman, J. L. (2018). Breathing matters. Nature Reviews Neuroscience, 19(6), 351–367. https://doi.org/10.1038/s41583-018-0003-6
Diekman, C. O., Thomas, P. J., & Wilson, C. G. (2017). Eupnea, tachypnea, and autoresuscitation in a closed-loop respiratory control model. Journal of Neurophysiology, 118(4), 2194–2215. https://doi.org/10.1152/jn.00170.2017
Dunmyre, J. R., Del Negro, C. A., & Rubin, J. E. (2011). Interactions of persistent sodium and calcium-activated nonspecific cationic currents yield dynamically distinct bursting regimes in a model of respiratory neurons. Journal of Computational Neuroscience, 31(2), 305–328. https://doi.org/10.1007/s10827-010-0311-y
Erhardt, A. H., Mardal, K. A., & Schreiner, J. E. (2020) Dynamics of a neuron–glia system: the occurrence of seizures and the influence of electroconvulsive stimuli: A mathematical and numerical study. Journal of Computational Neuroscience, 48(2), 229–251. https://doi.org/10.1007/s10827-020-00746-5
Ermentrout, B. (2002). Simulating, Analyzing, and Animating Dynamical Systems: A Guide To Xppaut for Researchers and Students. Society for Industrial and Applied Mathematics, USA.
Ermentrout, G. B., & Kopell, N. (1986). Parabolic bursting in an excitable system coupled with a slow oscillation. SIAM Journal on Applied Mathematics, 46(2), 233–253. https://doi.org/10.1137/0146017
Ezure, K., Tanaka, I., & Saito, Y. (2003). Activity of brainstem respiratory neurones just before the expiration?inspiration transition in the rat. Journal of Physiology, 547(2), 629–640. https://doi.org/10.1113/jphysiol.2002.032805
Fröhlich, F., Bazhenov, M., Iragui-Madoz, V., & Sejnowski, T. J. (2008). Potassium dynamics in the epileptic cortex: new insights on an old topic. Neuroscientist, 14(5), 422–433. https://doi.org/10.1177/1073858408317955
Gray, P. A., Rekling, J. C., Bocchiaro, C. M., & Feldman, J. L. (1999). Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science, 286(5444), 1566–1568. https://doi.org/10.1126/science.286.5444.1566
Hayes, J. A., Mendenhall, J. L., Brush, B. R., & Del Negro, C. A. (2008). 4-Aminopyridine-sensitive outward currents in preBötzinger complex neurons influence respiratory rhythm generation in neonatal mice. Journal of Physiology, 586(7), 1921–1936. https://doi.org/10.1113/jphysiol.2008.150946
He, L. S., Rue, M. C., Morozova, E. O., Powell, D. J., James, E. J., Kar, M., & Marder, E. (2020). Rapid adaptation to elevated extracellular potassium in the pyloric circuit of the crab, Cancer borealis. Journal of Neurophysiology, 123(5), 2075–2089. https://doi.org/10.1152/jn.00135.2020
Hodgkin, A., & Huxley, A. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117(4), 500–544. https://doi.org/10.1113/jphysiol.1952.sp004764
Huang, S., Hong, S., & De Schutter, E. (2015). Non-linear leak currents affect mammalian neuron physiology. Frontiers in Cellular Neuroscience, 9(432), 1–10. https://doi.org/10.3389/fncel.2015.00432
Huguenard, J. R., & McCormick, D. A. (1992). Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. Journal of Neurophysiology, 68(4), 1373–1383. https://doi.org/10.1152/jn.1992.68.4.1373
Izhikevich, E. M. (2007) Dynamical Systems in Neuroscience: The Geometry of Excitability and Bursting. Massachusetts Institute of Technology, Cambridge, MA.
Jasinski, P. E., Molkov, Y. I., Shetsova, N. A., Smith, J. C., & Rybak, I. A. (2013). Sodium and calcium mechanisms of rhythmic bursting in excitatory neural networks of the pre-Bštzinger complex: a computational modelling study.European Journal of Neuroscience, 37(2), 212–230. https://doi.org/10.1111/ejn.12042
Johnson, S. M., Smith, J. C., Funk, G. D., & Feldman, J. L. (1994). Pacemaker behavior of respiratory neurons in medullary slices from neonatal rat. Journal of Neurophysiology, 72(6), 2598–2608. https://doi.org/10.1152/jn.1994.72.6.2598
Kallurkar, P. S., Grover, C., Picardo, M. C. D., & Del Negro, C. A. (2020). Evaluating the burstlet theory of inspiratory rhythm and pattern generation. eNeuro, 7(1), 1–12. https://doi.org/doi.org/10.1523/ENEURO.0314-19.2019
Kam, K., Worrell, J. W., Janczewski, W. A., Cui, Y., & Feldman, J. L. (2013). Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. Journal of Neuroscience, 33(22), 9235–9245. https://doi.org/10.1523/JNEUROSCI.4143-12.2013
Kofuji, P., & Newman, E. A. (2004). Potassium buffering in the central nervous system. Neuroscience, 129(4), 1043–1054. https://doi.org/10.1016/j.neuroscience.2004.06.008
Shella Keilholz Koizumi, H., & Smith, J. C. (2008). Persistent Na\(^{+}\) and K\(^{+}\)- dominated leak currents contribute to respiratory rhythm generation in the pre-Bötzinger complex in vitro. Journal of Neuroscience, 28(7), 1773–1785. https://doi.org/10.1523/JNEUROSCI.3916-07.2008
Koizumi, H., Smerin, S. E., Yamanishi, T., Moorjani, B. R., Zhang, R., & Smith, J. C. (2010). TASK channels contribute to the K\(^{+}\)-dominated leak current regulating respiratory rhythm generation in vitro. Journal of Neuroscience, 30(12), 4273–4284. https://doi.org/10.1523/JNEUROSCI.4017-09.2010
Koizumi, H., John, T. T., Chia, J. X., Tariq, M. F., Phillips, R. S., Mosher, B., Chen, Y., Thompson, R., Zhang, R., Koshiya. N., & Smith, J. C. (2018). Transient receptor potential channels TRPM4 and TRPC3 critically contribute to respiratory motor pattern formation but not rhythmogenesis in rodent brainstem circuits. eNeuro, 5(1), 1–22. https://doi.org/10.1523/ENEURO.0332-17.2018
Krey, R. A., Goodreau, A. M., Arnold, T. B., & Del Negro, C. A. (2010). Outward currents contributing to inspiratory burst termination in preBötzinger Complex neurons of neonatal mice studied in vitro. Frontiers in Neural Circuits, 4(124), 1–9. https://doi.org/10.3389/fncir.2010.00124
Kueh, D., Barnett, W. H., Cymbalyuk, G. S., & Calabrese, R. L. (2016). Na\(^{+}\)/K\(^{+}\) pump interacts with the h-current to control bursting activity in central pattern generator neurons of leeches. eLife 5:e19322. https://doi.org/10.7554/eLife.19322
Lindsey, B. G., Rybak, I. A., & Smith, J. C. (2012). Computational models and emergent properties of respiratory neural networks. Comprehensive Physiology, 2(3), 1619–1670. https://doi.org/10.1002/cphy.c110016
Molkov, Y. I., Shevtsova, N. A., Park, C., Ben-Tal, A., Smith. J. C., Rubin, J. E., & Rybak, I. A. (2014). A closed-loop model of the respiratory system: focus on hypercapnia and active expiration. PloS One, 9(10), e109894. https://doi.org/10.1371/journal.pone.0109894
Molkov, Y. I., Rubin, J. E., Rybak, I. A., & Smith, J. C. (2017). Computational models of the neural control of breathing. Wiley Interdisciplinary Reviews: Systems Biology and Medicine, 9(2). https://doi.org/10.1002/wsbm.1371
Newman, E., & Reichenbach, A. (1996). The Müller cell: a functional element of the retina. Trends in Neurosciences, 19(8), 307–312. https://doi.org/10.1016/0166-2236(96)10040-0
Nicholson, C., & Syková. E. (1998). Extracellular space structure revealed by diffusion analysis. Trends in Neurosciences, 21(5), 207–215. https://doi.org/10.1016/s0166-2236(98)01261-2
Okada, Y., Kuwana, S., Kawai, A., Mückenhoff, K., & Scheid, P. (2005). Significance of extracellular potassium in central respiratory control studied in the isolated brainstem–spinal cord preparation of the neonatal rat. Respiratory Physiology & Neurobiology, 146(1), 21–32. https://doi.org/10.1016/j.resp.2004.10.009
Pace, R. W., Mackay, D. D., Feldman, J. L., & Del Negro, C. A. (2007). Inspiratory bursts in the preBötzinger complex depend on a calcium–activated non–specific cation current linked to glutamate receptors in neonatal mice. Journal of Physiology, 582(1), 113–125. https://doi.org/10.1113/jphysiol.2007.133660
Peña, F., Parkis, M. A., Tryba, A. K., & Ramirez, J. M. (2004). Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron, 43(1), 105–117. https://doi.org/10.1016/j.neuron.2004.06.023
Phillips, R. S., & Rubin, J. E. (2019). Effects of persistent sodium current blockade in respiratory circuits depend on the pharmacological mechanism of action and network dynamics. PLoS Computational Biology, 15(8), e1006938. https://doi.org/10.1371/journal.pcbi.1006938
Phillips, R. S., John, T. T., Koizumi, H., Molkov, Y. I., & Smith, J. C. (2019). Biophysical mechanisms in the mammalian respiratory oscillator re-examined with a new data-driven computational model. eLife 8:e41555. https://doi.org/10.7554/eLife.41555
Phillips, W. S., Del Negro, C. A., & Rekling, J. C. (2018). Dendritic A-current in rhythmically active preBötzinger complex neurons in organotypic cultures from newborn mice. Journal of Neuroscience, 38(12), 3039–3049. https://doi.org/10.1523/JNEUROSCI.3342-17.2018
Picardo, M. C. D., Sugimura, Y. K., Dorst, K. E., Kallurkar, P. S., Akins, V. T., Ma, X., Teruyama, R., Guinamard, R., Kam, K., Saha, M. S., & Del Negro, C. A. (2019). TRPM4 ion channels in pre-Bötzinger complex interneurons are essential for breathing motor pattern but not rhythm. PLoS Biology, 17(2), e2006094. https://doi.org/10.1371/journal.pbio.2006094
Purvis, L. K., Smith, J. C., Koizumu, H., & Butera, R. J. (2007). Intrinsic bursters increase the robustness of rhythm generation in an excitatory network. Journal of Neurophysiology, 97(2), 1515–1526. https://doi.org/10.1152/jn.00908.2006
Raimondo, J. V., Burman, R. J., Katz, A. A., & Akerman, C. J. (2015). Ion dynamics during seizures. Frontiers in Cellular Neuroscience, 9(419), 1–14. https://doi.org/10.3389/fncel.2015.00419
Ransdell, J. L., Nair, S. S., & Schulz, D. J. (2012). Rapid homeostatic plasticity of intrinsic excitability in a central pattern generator network stabilizes functional neural network output. Journal of Neuroscience, 32(28), 9649–9658. https://doi.org/10.1523/JNEUROSCI.1945-12.2012
Rekling, J. C., & Feldman, J. L. (1998). PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annual Review of Physiology, 60(1), 385–405. https://doi.org/10.1146/annurev.physiol.60.1.385
Richter, D. W. (1996). Neural regulation of respiration: rhythmogenesis and afferent control. In R. Greger, & U. Windhorst (Eds.), Comprehensive human physiology (pp. 2079–2095). Springer, Berlin, Heidelberg.
Rinzel, J. (1987). A formal classification of bursting mechanisms in excitable systems. In E. Teramoto, & M. Yamoguti (Eds.), Mathematical topics in population biology, morphogenesis and neurosciences (pp. 267–281), Springer-Verlag, New York, NY.
Rubin, J. E., Krauskopf, B., & Osinga, H. (2018). Natural extension of fast–slow decomposition for dynamical systems. Physical Review E, 97(1), 012215. https://doi.org/10.1103/PhysRevE.97.012215
Rubin, J. E., & Smith, J. C. (2019). Robustness of respiratory rhythm generation across dynamic regimes. PLoS Computational Biology, 15(7), e1006860. https://doi.org/10.1371/journal.pcbi.1006860
Rubin, J. E., Hayes, J. A., Mendenhall. J. L., & Del Negro, C. A. (2009) Calcium-activated nonspecific cation current and synaptic depression promote dependent burst oscillators. Proceedings of the National Academy of Sciences, 106(8), 2939–2944. https://doi.org/10.1073/pnas.0808776106
Rubin, J. E., Shevtsova, N. A., Ermentrout, G. B., Smith, J. C., & Rybak, I. A. (2009). Multiple rhythmic states in a model of the respiratory central pattern generator. Journal of Neurophysiology, 101(4), 2146–2165. https://doi.org/10.1152/jn.90958.2008
Rybak, I. A., Abdala, A. P. L., Markin, S. N., Paton, J. F. R., & Smith, J. C. (2007). Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Progress in Brain Research, 165, 201–220. https://doi.org/10.1016/S0079-6123(06)65013-9
Smith, J. C., Ellenberger, H. H., Ballanyi, K., Richter, D. W., & Feldman, J. L. (1991). Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science, 254(5032), 726–729. https://doi.org/10.1126/science.1683005
Somjen, G. G. (2004) Ions in the Brain: Normal Function, Seizures, and Stroke. Oxford University Press, New York, NY.
Teka, W., Tabak, J., & Bertram, R. (2012). The relationship between two fast/slow analysis techniques for bursting oscillations. Chaos: An Interdisciplinary Journal of Nonlinear Science, 22(4), 043117. https://doi.org/10.1063/1.4766943
Thoby-Brisson, M., & Ramirez, J. M. (2001). Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. Journal of Neurophysiology, 86(1), 104–112. https://doi.org/10.1152/jn.2001.86.1.104
Toporikova, N., Chevalier, M., & Thoby-Brisson, M. (2015). Sigh and eupnea rhythmogenesis involve distinct interconnected subpopulations: a combined computational and experimental study. eNeuro, 2(2), 1–18. https://doi.org/10.1523/ENEURO.0074-14.2015
Tryba, A. K., Peña, F., & Ramirez, J. M. (2003). Stabilization of bursting in respiratory pacemaker neurons. Journal of Neuroscience, 23(8), 3538–3546. https://doi.org/10.1523/JNEUROSCI.23-08-03538.2003
Vo, T., Tabak, J., Bertram, R., & Wechselberger, M. (2014). A geometric understanding of how fast activating potassium channels promote bursting in pituitary cells. Journal of Computational Neuroscience, 36(2), 259–278. https://doi.org/10.1007/s10827-013-0470-8
Wang, J., Lu, B., Liu, S., & Jiang, X. (2017). Bursting types and bifurcation analysis in the pre-Bötzinger complex respiratory rhythm neuron. International Journal of Bifurcation and Chaos, 27(1), 1750010. https://doi.org/10.1142/S0218127417500109
Wang, Y., & Rubin, J. E. (2016). Multiple timescale mixed bursting dynamics in a respiratory neuron model. Journal of Computational Neuroscience, 41(3), 245–268. https://doi.org/10.1016/j.mbs.2016.07.003
Wang, Y., & Rubin, J. E. (2020). Complex bursting dynamics in an embryonic respiratory neuron model. Chaos: An Interdisciplinary Journal of Nonlinear Science, 30(4), 043127. https://doi.org/10.1063/1.5138993
Zavala-Tecuapetla, C., Aguileta, M., Lopez-Guerrero, J., González-Marín, M., & Peña, F. (2008). Calcium-activated potassium currents differentially modulate respiratory rhythm generation. European Journal of Neuroscience, 27(11), 2871–2884. https://doi.org/10.1111/j.1460-9568.2008.06214.x
Acknowledgements
The authors would like to acknowledge the Program in Neural Computation at the Center for the Neural Basis of Cognition for their help in facilitating this research collaboration
Funding
This work was partially supported by NSF awards DMS-1612913 and DMS-1950195 to JER. Additional funding was provided by the University of Florida through the Wentworth Travel Scholarship and the University Scholars Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to disclose.
Additional information
Communicated by Action Editor: David Terman.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
1.1 Constants and parameters
The complete list of parameters used for this model is shown below. Certain parameters were fixed for all simulations, while others were varied for different tests. These instances will be noted.
Universal & Experimental Constants:
-
Elementary Charge: \(q=1.602 \times 10^{-19}\) C.
-
Avogadro Constant: \(N_{A} = 6.022 \times 10^{23}\) \(\frac{1}{mol}\).
-
Unit Time Constant: \(\tau = 1000\) \(\frac{ms}{s}\).
-
Ratio of Volumes: \(\beta = 14.555\) (modified from Barreto and Cressman (2011), \(\beta = 7\)).
-
Membrane Capacitance: \(C = 36\) pF (taken from Rybak et al. (2007)).
Derived Constants:
-
Current Conversion Constant: \(\gamma = 7.214 \times 10^{-3}\) \(\frac{mM}{s \cdot pA}\) (derived in Appendix 2).
Maximal Conductances:
-
\(\bar{g}_{Na} = 150\) nS (taken from Jasinski et al. (2013)).
-
\(\bar{g}_{NaP} = 5\) nS (taken from Bacak et al. (2016b)). Varied as parameter in Sect. 4.
-
\(\bar{g}_{K} = 160\) nS (taken from Jasinski et al. (2013)).
-
\(\bar{g}_{L} = 2.5\) nS (taken from Jasinski et al. (2013), \(\bar{g}_{L} \in [ 2,3 ]\)). Varied as parameter in Sect. 4.
-
\(\bar{g}_{Syn} = 0.365\) nS. (Introduced in this paper to represent constant synaptic drive, in contrast to model in Bacak et al. (2016b) where \(\bar{g}_{Syn} = 0\)). Varied as parameter in Sect. 4.
Ion Concentrations & Reversal Potentials:
-
\([Na^{+}]_{out}=120\) mM (taken from Jasinski et al. (2013)).
-
\([Na^{+}]_{in}=15\) mM (taken from Izhikevich (2007), \([Na^{+}]_{in} \in [5, 15]\) mM).
-
\(E_{Na} = 26.7 \cdot \log {\frac{[Na^{+}]_{out}}{[Na^{+}]_{in}}} = 55.5\) mV. (Consistent with Rybak et al. (2007), \(E_{Na} = 55\) mV).
-
\([K^{+}]_{in} = 160\) mM (modified from Izhikevich (2007); Jasinski et al. (2013), \([K^{+}]_{in} = 140\) mM).
-
\(E_{L} = -68\) mV (taken from Jasinski et al. (2013)).
-
\(E_{Syn} = -10\) mV (taken from Jasinski et al. (2013)).
Parameters for Fast Sodium \((I_{Na})\) and Persistent Sodium (\(I_{NaP}\)):
-
\(V_{m_{Na}} = -43.8\) mV, \(k_{m_{Na}} = 6\) mV, \(V_{\tau _{m_{Na}}} = -43.8\) mV, \(k_{\tau _{m_{Na}}} = 14\) mV.
-
\(V_{h_{Na}} = -67.5\) mV, \(k_{h_{Na}} = -11.8 mV\), \(V_{\tau _{h_{Na}}} = -67.5\) mV, \(k_{\tau _{h_{Na}}} = -12.8\) mV.
-
\(V_{m_{NaP}} = -47.1\) mV, \(k_{m_{NaP}} = 3.1\) mV, \(V_{\tau _{m_{NaP}}} = -47.1\) mV, \(k_{\tau _{m_{NaP}}} = 6.2\) mV.
-
\(V_{h_{NaP}} = -60\) mV, \(k_{h_{NaP}} = -9\) mV, \(V_{\tau _{h_{NaP}}} = -60\) mV, \(k_{\tau _{h_{NaP}}} = 9\) mV.
-
\(\bar{\tau }_{m_{Na}} =0.25\) mS, \(\bar{\tau }_{h_{Na}} = 8.46\) mS, \(\bar{\tau }_{m_{NaP}} =1\) mS, \(\bar{\tau }_{h_{NaP}} = 5000\) mS.
-
All of these parameters were taken directly from Bacak et al. (2016a), with the exception of \(k_{h_{Na}}\), which was altered from a value of \(-10.8\) mV to the listed value of \(-11.8\) mV.
Parameters for Delayed Rectifier Potassium Current \((I_{K})\):
-
\(n_{A} = 0.01\) \(\frac{1}{mV}\), \(n_{A_{V}} = 44\) mV, \(n_{A_{k}} = 5\) mV, \(n_{B} = 0.17\), \(n_{B_{V}} = 49\) mV, \(n_{B_{k}} = 40\) mV.
-
All values taken from Bacak et al. (2016b).
Parameters for Diffusion of Extracellular Potassium \(([K^{+}]_{out})\):
-
\(k_{bath}=4\) mM (taken from Barreto and Cressman (2011)).
-
\(\tau _{diff} = 750\) mS (numerically equivalent to the formulation in Barreto and Cressman (2011), which uses \(\frac{1}{\tau _{diff}} \equiv \frac{\epsilon }{\tau }\), where \(\epsilon =1.333\) Hz and \(\tau =1000 \frac{\mathrm{mS}}{\mathrm{s}}\)).
Parameters for Glia:
-
\(\bar{G} = 10 \frac{mM}{s}\), \(\bar{K} = 5 mM\), \({z_{K}} = 6 \frac{1}{mM}\).
-
These parameter values were altered from those in Barreto and Cressman (2011). In Barreto and Cressman (2011), the concentration of \([K^{+}]_{out}\) remains far below the mid-point value of the sigmoidal function in Eq. (10). The parameters were adjusted such that the range of dynamic \([K^{+}]_{out}\) was distributed over the midpoint of Eq. (10), ensuring that the nonlinear behavior of glial cells was represented.
Appendix 2
1.1 Derivation of \(\gamma\)
Our initial assumption is that the neuron is roughly spherical, or rather that the majority of the cell’s volume is contained in a sphere. From Barreto and Cressman (2011), the radius of the neuron is taken to be approximately \(r=7.0\) \(\mu \mathrm{m}\). Hence, the internal volume of the neuron can be approximated as \(V_{in} = \frac{4}{3} \pi r^3 = 1.44 \times 10^{-9}\) mL.
The internal concentration \(c_{in}\) can be determined from the total number of ions N, the internal volume \(V_{in}\), and Avogadro’s Constant \(N_{A}\):
Note that the ions we are measuring concentrations of are \(Na^{+}\) and \(K^{+}\), both of which have a \(+1\) charge. Letting \(q=1.60 \times 10^{-19}\) C, we can express the concentration in terms of total charge, Q:
Differentiating, we get:
By taking the ratio of this expression to the current, we can determine:
By the following dimensional analysis manipulation, we obtain:
Thus, we conclude:
Appendix 3
1.1 A closer look at transitions in behavior as \(E_{K}\) is varied
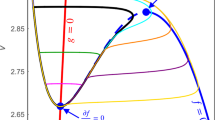
As illustrated in Fig. 1, if the \(K^{+}\) concentration is held fixed, then shifting the \(E_{K}\) value has a clear effect on the long-term periodic behavior of the model neuron. Each periodic behavior, whether tonic spiking or bursting, can be depicted as a stable limit cycle projected to the (\(h_{NaP},V\)) phase space. As shown in Fig. 3, with increases in \(E_K\), the stable oscillation switches from tonic spiking to bursting, and then, with additional increases, from bursting back to spiking. Which behavior arises depends on whether the periodic orbit family of the fast subsystem terminates in a SNIC bifurcation or a homoclinic bifurcation and on where this termination lies relative to the \(h_{NaP}\) nullcline.
Here we construct a bifurcation diagram to present in more detail the changes in stable periodic behavior that occur with \(E_{K}\) as a bifurcation parameter. More specifically, when the neuronal system exhibits bursting, each burst is composed of a finite number of action potentials, each associated with an approximately constant \(V, h_{NaP}\). Therefore, for each fixed \(h_{NaP}\), we identify the corresponding periodic spiking or bursting attractor and record the \(h_{NaP}\) value at which each spike occurs within this attractor (Fig. 8).
Bifurcation diagram of attracting dynamics of the neuronal model with \([K ^{+}]_{out}\) (and hence \(E_{K}\)) used as the bifurcation parameter, varied in steps of 0.001 mV. (A) Bifurcation diagram over the entire bursting interval. Each blue dot represents an \(h_{NaP}\) value on a single spike within the attractor for the corresponding \(E_K\). Insets show voltage and \(h_{NaP}\) time courses at the fixed values of \(E_K\) marked by the numbered vertical dashed lines on the diagram. (B-C) Zoomed views of different parts of the diagram in (A)
In this bifurcation diagram, each dot denotes the value of \(h_{NaP}\) at which an action potential occurs during 80 seconds of simulated bursting behavior, for a corresponding fixed value of \(E_K\). For each \(E_K\), the spikes from the first 55 seconds of neuron simulation are not shown, such that the diagram omits the transient state and only reflects the attractors of the system. For sufficiently low \(E_K\), the stable dynamics consists of periodic tonic spiking, characterized by a single \(h_{NaP}\) value for each \(E_K\) in the diagram. As \(E_K\) increases, the transition from a tonic spiking state to a bursting state appears to arise through a chaotic period doubling mechanism (Fig. 8A,B), estimated numerically to occur just above \(E_K = -91.2\) mV.
The transition from bursting back to tonic spiking, depicted in Fig. 8A,C, is less clear cut. The spike branch at highest \(h_{NaP}\) values seems to disappear instantly as \(E_K\) increases. We expect that this change is related to the phenomena shown in Figs. 1C, 3D. In the solution displayed in Fig. 1C, it appears that bursting is about to begin, but instead a plateau of depolarization block occurs. From Fig. 3D, we can appreciate that the AH point has moved to smaller \(h_{NaP}\) than that of the fold point, such that the trajectory’s initial jump to the active phase does not yield a full spike. Only after \(h_{NaP}\) drifts to lower values, below the AH point, can spiking ensue. With an additional increase in \(E_K\) to just below \(-83.1\) mV, most of the remaining spike branches disappear together, leaving only a cluster of values near \(h_{NaP}=0.155\). We also notice pockets of variability in \(h_{NaP}\) as \(E_K\) varies between \(-83.4\) and \(-83.1\) mV. Interestingly, inspection of the voltage trace suggests that periodic spiking begins at about \(E_K = -82.6\) mV, above the value at which most of the collection of \(h_{NaP}\) branches disappears.
Elucidating the details of this bifurcation is beyond the scope of our consideration of ramping bursts in the full model and remains for future inquiry, which would require more detailed simulations and analysis.
Appendix 4
1.1 1-fast-2-slow Analysis
The dynamics of ramping bursts can be understood through a fast-slow decomposition analysis. The first step was the fast-slow decomposition analysis discussed in Sect. 3.2. As shown in Fig. 3, this analysis involved fixing \([K^{+}]_{out}\) and determining the geometry that governs the model trajectory in the \(\left( V, h_{NaP} \right)\)-phase space. This analysis can naturally be extended into a three-dimensional fast-slow decomposition by including \([K^{+}]_{out}\), or equivalently \(E_K\), as a second slow variable. The various important geometric objects identified with fixed \([K^{+}]_{out}\) values, namely critical manifolds, fast subsystem saddle-node and Andronov-Hopf (AH) bifurcation points, and corresponding fast subsystem periodic orbits become higher dimensional surfaces and curves when projected to the \(\left( V, h_{NaP}, E_K \right)\)-phase space. For instance, the individual AH points become an AH curve, and the periodic orbits initiated there form a smooth manifold originating from this curve. A visualization of this three-dimensional structure is illustrated in Fig. 9, along with the superimposed trajectory of a neuron with dynamic \([K^{+}]_{out}\) exhibiting ramping bursts.
The periodic trajectory of the ramping burst solution in \((V, h_{NaP}, E_{K})\)-space is color coded temporally, progressing in time from blue to yellow. Additionally, the two black arrows indicate the direction that the trajectory travels. The upper and lower blue surfaces represent stable components of the critical manifold; white/blue surfaces between these are unstable components, which meet in a saddle-node curve at negative \(h_{NaP}\) (not shown). Also shown are the AH curve (red) and the surfaces of maximal and minimal V along the family of periodic orbits originating at the AH curve (green)
The period during which spiking occurs or active phase of the burst occurs where the trajectory oscillates between the prongs of the green periodic orbit manifold in Fig. 9. As discussed in Sect. 3.3, this oscillation drives build-up of \([K^{+}]_{out}\), moving the trajectory in the direction of increasing \(E_{K}\). This potassium ion build-up causes an increase in spiking frequency within the burst, facilitating further external potassium accumulation through a positive feedback loop. This process continues until the trajectory reaches a point along the homoclinic curve where the periodic orbit family terminates and returns to the hyperpolarized stable component of the critical manifold. This corresponds to the quiescent phase of the burst, where the trajectory remains until it reaches the saddle-node curve where it returns to the active phase. Additional insight arises from visualizing the local minima and maxima that occur throughout the active phase of the burst. In Fig. 10 these local extrema are connected into two curves. Clearly, the trajectory of the neuron travels along the family of periodic orbits during the burst, moving away from and back towards the homoclinic curve as time advances and \(E_K\) increases. Furthermore, the neuron experiences a decline in spike amplitude when it pulls away from the edge of the periodic orbit family where it starts and terminates.
Once again, the red curve indicates fast subsystem AH points and the green manifold consists of extremal voltages of periodic orbits emanating from the AH curve. Superimposed on this manifold are traces of minimum (lower, orange) and maximum (upper, red-orange) values of voltage attained for each spike within the burst, connected into smooth curves
Rights and permissions
About this article
Cite this article
Abdulla, M.U., Phillips, R.S. & Rubin, J.E. Dynamics of ramping bursts in a respiratory neuron model. J Comput Neurosci 50, 161–180 (2022). https://doi.org/10.1007/s10827-021-00800-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-021-00800-w