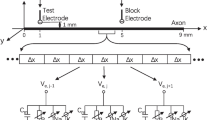
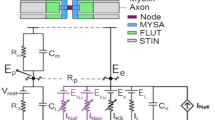
Abstract
Electrical stimulation of nerve fibers is used as a therapeutic tool to treat neurophysiological disorders. Despite efforts to model the effects of stimulation, its underlying mechanisms remain unclear. Current mechanistic models quantify the effects that the electrical field produces near the fiber but do not capture interactions between action potentials (APs) initiated by stimulus and APs initiated by underlying physiological activity. In this study, we aim to quantify the effects of stimulation frequency and fiber diameter on AP interactions involving collisions and loss of excitability. We constructed a mechanistic model of a myelinated nerve fiber receiving two inputs: the underlying physiological activity at the terminal end of the fiber, and an external stimulus applied to the middle of the fiber. We define conduction reliability as the percentage of physiological APs that make it to the somatic end of the nerve fiber. At low input frequencies, conduction reliability is greater than 95% and decreases with increasing frequency due to an increase in AP interactions. Conduction reliability is less sensitive to fiber diameter and only decreases slightly with increasing fiber diameter. Finally, both the number and type of AP interactions significantly vary with both input frequencies and fiber diameter. Modeling the interactions between APs initiated by stimulus and APs initiated by underlying physiological activity in a nerve fiber opens opportunities towards understanding mechanisms of electrical stimulation therapies.






Similar content being viewed by others
References
Agarwal, R., & Sarma, S.V. (2012). Performance limitations of relay neurons. PLoS computational biology, 8 (8), e1002,626.
Bair, W., Koch, C., Newsome, W., Britten, K. (1994). Power spectrum analysis of bursting cells in area mt in the behaving monkey. Journal of Neuroscience, 14(5), 2870–2892.
Barolat, G., Zeme, S., Ketcik, B. (1991). Multifactorial analysis of epidural spinal cord stimulation. Stereotactic and functional neurosurgery, 56(2), 77–103.
Baron, R. (2009). Neuropathic pain: a clinical perspective. In Canning, B.J., & Spina, D. (Eds.) Sensory Nerves (pp. 3–30). Springer.
Bruns, T.M., Bhadra, N., Gustafson, K.J. (2009). Bursting stimulation of proximal urethral afferents improves bladder pressures and voiding. Journal of Neural Engineering, 6(6), 1–8.
Crago, P.E., & Makowski, N.S. (2014). Alteration of neural action potential patterns by axonal stimulation: the importance of stimulus location. Journal of Neural Engineering, 11(5), 1–9.
De Luca, C.J., LeFever, R.S., McCue, M.P., Xenakis, A.P. (1982). Behaviour of human motor units in different muscles during linearly varying contractions. The Journal of Physiology, 329(1), 113–128.
Ehrens, D., Sritharan, D., Sarma, S.V. (2015). Closed-loop control of a fragile network: application to seizure-like dynamics of an epilepsy model. Frontiers in neuroscience, 9, 58.
Foutz, T.J., & McIntyre, C.C. (2010). Evaluation of novel stimulus waveforms for deep brain stimulation. Journal of neural engineering, 7(6), 1–10.
Frankenhaeuser, B., & Huxley, A.F. (1964). The action potential in the myelinated nerve fibre of Xenopus laevis as computed on the basis of voltage clamp data. The Journal of Physiology, 171(2), 302–315.
Grill, W.M., & Mortimer, J.T. (1998). Stability of the input-output properties of chronically implanted multiple contact nerve cuff stimulating electrodes. IEEE Transactions on Rehabilitation Engineering, 6(4), 364–373.
Groves, D.A., & Brown, V.J. (2005). Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neuroscience & Biobehavioral Reviews, 29(3), 493–500.
Hines, M.L., & Carnevale, N.T. (1997). The NEURON simulation environment. Neural Computation, 9(6), 1179–1209.
van den Honert, C., & Mortimer, J.T. (1981). A technique for collision block of peripheral nerve: Frequency dependence. IEEE Transactions on Biomedical Engineering, 5(28), 379–382.
Hursh, J.B. (1939). Conduction velocity and diameter of nerve fibers. American Journal of Physiology, 127 (1), 131–139.
Iggo, A. (1958). The electrophysiological identification of single nerve fibres, with particular reference to the slowest-conducting vagal afferent fibres in the cat. The Journal of Physiology, 142(1), 110–126.
Jänig, W, Grossmann, L., Gorodetskaya, N. (2009). Mechano-and thermosensitivity of regenerating cutaneous afferent nerve fibers. Experimental Brain Research, 196(1), 101–114.
Kajander, K.C., & Bennett, G.J. (1992). Onset of a painful peripheral neuropathy in rat: a partial and differential deafferentation and spontaneous discharge in a beta and a delta primary afferent neurons. Journal of Neurophysiology, 68(3), 734–744.
Katz, B. (1950). Action potentials from a sensory nerve ending. The Journal of Physiology, 111(3-4), 248.
Kralj, A.R., & Bajd, T. (1989). Functional electrical stimulation: standing and walking after spinal cord injury. Boca Raton: CRC Press.
McIntyre, C.C., & Grill, W.M. (1999). Model-based design of stimulus waveforms for selective microstimulation in the central nervous system. In Proceedings of the First Joint BMES/EMBS Conference, (Vol. 1 p. 384).
McIntyre, C.C., Richardson, A.G., Grill, W.M. (2002). Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. Journal of Neurophysiology, 87(2), 995–1006.
McNeal, D.R. (1976). Analysis of a model for excitation of myelinated nerve. IEEE Transactions on Biomedical Engineering, 23(4), 329–337.
Medtronic Neuromodulation. (2007). Technical design summary: Model 39565 specify 5-6-5 surgical lead.
Mortimer, J.T., & Bhadra, N. (2004). Peripheral nerve and muscle stimulation. In Neuroprosthetics: Theory and Practice, World Scientific (pp. 638–682).
Olin, J.C., Kidd, D.H., North, R.B. (1998). Postural changes in spinal cord stimulation perceptual thresholds. Neuromodulation: Technology at the Neural Interface, 1(4), 171–175.
Peckham, P.H., & Kilgore, K.L. (2013). Challenges and opportunities in restoring function after paralysis. IEEE Transactions on Biomedical Engineering, 60(3), 602–609.
Pfurtscheller, G., Müller, GR, Pfurtscheller, J., Gerner, H.J., Rupp, R. (2003). ‘Thought’–control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neuroscience Letters, 351(1), 33–36.
Reilly, J.P. (1989). Peripheral nerve stimulation by induced electric currents: exposure to time-varying magnetic fields. Medical and Biological Engineering and Computing, 27(2), 101–110.
Sacré, P, Sarma, S.V., Guan, Y., Anderson, W.S. (2015). Electrical neurostimulation for chronic pain: on selective relay of sensory neural activities in myelinated nerve fibers. In Proceeding of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 4705–4708).
Sadashivaiah, V. (2018). Modeling the interactions in a mammalian nerve fiber. https://github.com/vjysd/nerve-fiber-modeling.
Sadashivaiah, V., Sacré, P, Guan, Y., Anderson, W.S., Sarma, S.V. (2017). Modeling electrical stimulation of mammalian nerve fibers: a mechanistic versus probabilistic approach. In Proceeding of the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 3868–3871).
Sadashivaiah, V., Sacré, P, Guan, Y., Anderson, W.S., Sarma, S.V. (2018). Studying the interactions in a mammalian nerve fiber: a functional modeling approach. In Proceeding of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). https://doi.org/10.1109/EMBC.2018.8512975, https://ieeexplore.ieee.org/document/8512975 (pp. 3525–3528).
Sadashivaiah, V., Sacré, P, Guan, Y., Anderson, W.S., Sarma, S.V. (2018). Selective relay of afferent sensory-induced action potentials from peripheral nerve to brain and the effects of electrical stimulation. In Proceeding of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). https://doi.org/10.1109/EMBC.2018.8513029, https://ieeexplore.ieee.org/document/8513029 https://ieeexplore.ieee.org/document/8513029 (pp. 3606–3609).
Schwarz, J.R., Reid, G., Bostock, H. (1995). Action potentials and membrane currents in the human node of ranvier. Pflü,gers Archiv, 430(2), 283–292.
Shealy, C.N., Mortimer, J.T., Reswick, J.B. (1967). Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesthesia & Analgesia, 46(4), 489–491.
Shechter, R., Yang, F., Xu, Q., Cheong, Y.K., He, S.Q., Sdrulla, A., Carteret, A.F., Wacnik, P.W., Dong, X., Meyer, R.A., et al. (2013). Conventional and kilohertz-frequency spinal cord stimulation produces intensity-and frequency-dependent inhibition of mechanical hypersensitivity in a rat model of neuropathic pain. Anesthesiology, 119(2), 422–432.
Song, Y., Li, H.M., Xie, R.G., Yue, Z.F., Song, X.J., Hu, S.J., Xing, J.L. (2012). Evoked bursting in injured Aβ dorsal root ganglion neurons: a mechanism underlying tactile allodynia. Pain, 153(3), 657–665.
Stidd, D.A., Wuollet, A., Bowden, K., Price, T., Patwardhan, A., Barker, S., Weinand, M.E., Annabi, J., Annabi, E. (2012). Peripheral nerve stimulation for trigeminal neuropathic pain. Pain Physician, 15(1), 27–33.
Struijk, J.J., Holsheimer, J., Van Veen, B., Boom, H.B. (1991). Epidural spinal cord stimulation: calculation of field potentials with special reference to dorsal column nerve fibers. IEEE Transactions on Biomedical Engineering, 38(1), 104–110.
Tarler, M.D., & Mortimer, J.T. (2004). Selective and independent activation of four motor fascicles using a four contact nerve-cuff electrode. IEEE Transactions on neural systems and rehabilitation engineering, 12(2), 251–257.
Troy, J., & Robson, J. (1992). Steady discharges of x and y retinal ganglion cells of cat under photopic illuminance. Visual neuroscience, 9(6), 535–553.
Tyler, D.J., & Durand, D.M. (2002). Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 10(4), 294–303.
Wall, P.D., & Sweet, W.H. (1967). Temporary abolition of pain in man. Science, 155(3758), 108–109.
Wesselink, W.A., Holsheimer, J., Boom, H.B. (1999). A model of the electrical behaviour of myelinated sensory nerve fibres based on human data. Medical and Biological Engineering and Computing, 37(2), 228–235.
Yoshida, K., & Horch, K. (1993). Selective stimulation of peripheral nerve fibers using dual intrafascicular electrodes. IEEE transactions on biomedical engineering, 40(5), 492–494.
Acknowledgments
Work supported by NIH R01 AT009401 to S.V.S, Y.G., and W.S.A., and by NPRI postdoctoral fellowship awarded to P.S. We would like to thank Dr. Michael Caterina, Neurosurgery Pain Research Institute, The Johns Hopkins University School of Medicine for valuable and insightful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Gaute T. Einevoll
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
The fiber model and its parameters at 37°C Fiber geometry
- ᅟ:
-
axon diameter, d = 6–12 μm (step size of 3 μm)
- ᅟ:
-
ratio of axon to fiber diameter, rdD = 1
- ᅟ:
-
fiber diameter, D = d × rdD
- ᅟ:
-
ratio of li to fiber diameter, \(r_{\mathrm {l_{i}D}} = 100\)
- ᅟ:
-
internodal length, \(l_{i} = D \times r_{\mathrm {l_{i}D}}\)
- ᅟ:
-
fiber length, L = 10 cm
- ᅟ:
-
nodal length, l = 2.5 μm
- ᅟ:
-
number of nodes, \(n = \lceil 1 + \frac {L}{l + l_{i}}\rceil \)
Parameters
- ᅟ:
-
αmA = 1.86,αmB = 65.6,αmC = 10.3
- ᅟ:
-
αhA = 0.0336,αhB = − 27,αhC = 11.0
- ᅟ:
-
αnA = 0.00789,αnB = − 9.2,αnC = 1.10
- ᅟ:
-
αsA = 0.00122,αsB = 71.5,αsC = 23.6
- ᅟ:
-
βmA = 0.0860,βmB = 61.3,βmC = 9.16
- ᅟ:
-
βhA = 2.30,βhB = 55.2,βhC = 13.4
- ᅟ:
-
βnA = 0.0142,βnB = 8,βnC = 10.5
- ᅟ:
-
βsA = 0.000739,βsB = 3.9,βsC = 21.8
- ᅟ:
-
gating coefficients α∗A, β∗A in ms− 1
- ᅟ:
-
gating coefficients α∗B, β∗B, α∗C, β∗C in mV
- ᅟ:
-
sodium permeability, PNa = 7.04 × 10− 3 cm/s
- ᅟ:
-
potassium conductance (fast), gKf = 0.015 S/cm2
- ᅟ:
-
potassium conductance (slow), gKf = 0.030 S/cm2
- ᅟ:
-
leakage conductance, gL = 60 × 10− 3 S/cm2
- ᅟ:
-
sodium concentration outside, [Na]o = 154 mM
- ᅟ:
-
sodium concentration inside, [Na]i = 35 mM
- ᅟ:
-
potassium concentration outside, [K]o = 5.6 mM
- ᅟ:
-
potassium concentration inside, [K]i = 155 mM
- ᅟ:
-
sodium equilibrium potential, VNa = − 84 mV
- ᅟ:
-
potassium equilibrium potential, VK = + 60 mV
- ᅟ:
-
resting membrane potential, Vr = − 84 mV
- ᅟ:
-
Faraday constant, F = 96485 C/mol
- ᅟ:
-
gas constant, R = 8.3144 J/Kmol
- ᅟ:
-
absolute temperature, T = 310.15 K
- ᅟ:
-
membrane potential, V mV
Membrane currents
- ᅟ:
-
sodium current, INa mA/cm2
- ᅟ:
-
potassium current (fast), IKf mA/cm2
- ᅟ:
-
potassium current (slow), IKs mA/cm2
- ᅟ:
-
leakage current, IL mA/cm2
- ᅟ:
-
\(I_{Na} = m^{3}hP_{\text {Na}}\frac {VF^{2}}{RT}\frac {([\text {Na}]_{\mathrm {o}} - [\text {Na}]_{\mathrm {i}}e^{VF/RT})}{(1 - e^{VF/RT})}\)
- ᅟ:
-
IKf = n4gKf(V − VK)
- ᅟ:
-
IKs = sgKs(V − VK)
- ᅟ:
-
IL = gL(V − VL)
Simulation parameters
- ᅟ:
-
simulation duration, tstop = 30 s
- ᅟ:
-
simulation step size, tstep = 0.001 ms
- ᅟ:
-
simulation repeats, nrep = 50
Rights and permissions
About this article
Cite this article
Sadashivaiah, V., Sacré, P., Guan, Y. et al. Modeling the interactions between stimulation and physiologically induced APs in a mammalian nerve fiber: dependence on frequency and fiber diameter. J Comput Neurosci 45, 193–206 (2018). https://doi.org/10.1007/s10827-018-0703-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-018-0703-y