Abstract
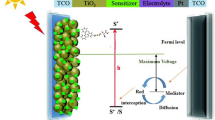
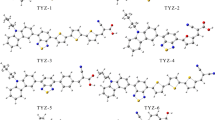
Density functional theory and time-dependent approaches are applied for theoretical investigation of a new class of novel carbazole-based d–D–π–A-type dyes, where the carbazole moiety is the main electron donor, bithiophene behaves as a π-bridge, and cyanoacetic acid as an electron acceptor for all the studied dyes, whereas the terminal electron donor unit is varied to thiophene, thienothiophene, carbazole, dimethoxyphenyl, and indole. The influence of the terminal electron donor on the optoelectronics properties is investigated for the dyes in isolated state and in chloroform solvent. Their absorption spectra and electronic and structural properties are evaluated and discussed. The theoretical results show that all the dyes exhibit excellent optoelectronic properties. In particular, D5 with indole as the terminal electron donor moiety has potential for use as a sensitizer for nanocrystalline TiO2 solar cells based on its red-shifted absorption spectrum, reduced energy gap, lowest λtotal value, and higher \(\Delta G^{\text{Inject}}\) and \(\Delta G^{\text{Reg}}\) values.







Similar content being viewed by others
References
O’Regan, B., Grätzel, M.: A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737 (1991)
Grätzel, M.: Photoelectrochemical cells. Nature 414, 338 (2001)
Hagfeldt, A., Boschloo, G., Sun, L., Kloo, L., Pettersson, H.: Dye-sensitized solar cells. Chem. Rev. 110, 6595–6663 (2010)
Gonçalves, L.M., de Zea Bermudez, V., Ribeiro, H.A., Mendes, A.M.: Dye-sensitized solar cells: a safe bet for the future. Energy Environ. Sci. 1, 655–667 (2008)
Chen, C.-Y., Wang, M., Li, J.-Y., Pootrakulchote, N., Alibabaei, L., Ngoc-le, C., Decoppet, J.-D., Tsai, J.-H., Grätzel, C., Wu, C.-G.: Highly efficient light-harvesting ruthenium sensitizer for thin-film dye-sensitized solar cells. ACS Nano 3, 3103–3109 (2009)
Mishra, A., Fischer, M.K.R., Bäuerle, P.: Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed. 48, 2474–2499 (2009)
Kuang, D., Walter, P., Nüesch, F., Kim, S., Ko, J., Comte, P., Zakeeruddin, S.M., Nazeeruddin, M.K., Grätzel, M.: Co-sensitization of organic dyes for efficient ionic liquid electrolyte-based dye-sensitized solar cells. Langmuir 23, 10906–10909 (2007)
Qin, P., Yang, X., Chen, R., Sun, L., Marinado, T., Edvinsson, T., Boschloo, G., Hagfeldt, A.: Influence of π-conjugation units in organic dyes for dye-sensitized solar cells. J. Phys. Chem. C 111, 1853–1860 (2007)
Mathew, S., Yella, A., Gao, P., Humphry-Baker, R., Curchod, B.F.E., Ashari-Astani, N., Tavernelli, I., Rothlisberger, U., Nazeeruddin, M.K., Grätzel, M.: Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 6, 242 (2014)
Cai, S., Tian, G., Li, X., Su, J., Tian, H.: Efficient and stable DSSC sensitizers based on substituted dihydroindolo [2, 3-b] carbazole donors with high molar extinction coefficients. J. Mater. Chem. A 1, 11295–11305 (2013)
Koumura, N., Wang, Z.-S., Mori, S., Miyashita, M., Suzuki, E., Hara, K.: Alkyl-functionalized organic dyes for efficient molecular photovoltaics. J. Am. Chem. Soc. 128, 14256–14257 (2006)
Hwang, S., Lee, J.H., Park, C., Lee, H., Kim, C., Park, C., Lee, M.-H., Lee, W., Park, J., Kim, K.: A highly efficient organic sensitizer for dye-sensitized solar cells. Chem. Commun. (46), 4887–4889 (2007)
Liang, M., Chen, J.: Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 42, 3453–3488 (2013)
Cai, L., Moehl, T., Moon, S.-J., Decoppet, J.-D., Humphry-Baker, R., Xue, Z., Bin, L., Zakeeruddin, S.M., Grätzel, M.: 4, 9-Dihydro-4, 4, 9, 9-tetrahexyl-s-indaceno [1, 2-b: 5, 6-b′] dithiophene as a π-spacer of donor − π–acceptor dye and its photovoltaic performance with liquid and solid-state dye-sensitized solar cells. Org. Lett. 16, 106–109 (2013)
Liu, J., Yang, X., Islam, A., Numata, Y., Zhang, S., Salim, N.T., Chen, H., Han, L.: Efficient metal-free sensitizers bearing circle chain embracing π-spacers for dye-sensitized solar cells. J. Mater. Chem. A 1, 10889–10897 (2013)
Kim, J.Y., Kim, Y.H., Kim, Y.S.: Indoline dyes with various acceptors for dye-sensitized solar cells. Curr. Appl. Phys. 11, S117–S121 (2011)
Liu, B., Wu, W., Li, X., Li, L., Guo, S., Wei, X., Zhu, W., Liu, Q.: Molecular engineering and theoretical investigation of organic sensitizers based on indoline dyes for quasi-solid state dye-sensitized solar cells. Phys. Chem. Chem. Phys. 13, 8985–8992 (2011)
Liu, B., Li, W., Wang, B., Li, X., Liu, Q., Naruta, Y., Zhu, W.: Influence of different anchoring groups in indoline dyes for dye-sensitized solar cells: electron injection, impedance and charge recombination. J. Power Sour. 234, 139–146 (2013)
Cho, M.J., Park, S.S., Yang, Y.S., Kim, J.H., Choi, D.H.: Molecular design of donor–acceptor-type cruciform dyes for efficient dyes-sensitized solar cells. Synth. Met. 160, 1754–1760 (2010)
Ding, W.-L., Wang, D.-M., Geng, Z.-Y., Zhao, X.-L., Xu, W.-B.: Density functional theory characterization and verification of high-performance indoline dyes with D–A–π–A architecture for dye-sensitized solar cells. Dyes Pigments 98, 125–135 (2013)
Zhao, Z., Xu, X., Wang, H., Lu, P., Yu, G., Liu, Y.: Zigzag molecules from pyrene-modified carbazole oligomers: synthesis, characterization, and application in OLEDs. J. Org. Chem. 73, 594–602 (2008)
Li, T., Gao, J., Cui, Y., Zhong, C., Ye, Q., Han, L.: Novel D–π–A carbazole sensitizers with 4-phenyl-2-(thiophen-2-yl) thiazole as π-bridge for dye-sensitized solar cells. J. Photochem. Photobiol. A 303, 91–98 (2015)
Liu, D., Fessenden, R.W., Hug, G.L., Kamat, P.V.: Dye capped semiconductor nanoclusters. Role of back electron transfer in the photosensitization of SnO2 nanocrystallites with cresyl violet aggregates. J. Phys. Chem. B 101, 2583–2590 (1997)
Narayan, M.R.: Dye sensitized solar cells based on natural photosensitizers. Renew. Sustain. Energy Rev. 16, 208–215 (2012)
Wang, J., Cong, S., Wen, S., Yan, L., Su, Z.: A rational design for dye sensitizer: density functional theory study on the electronic absorption spectra of organoimido-substituted hexamolybdates. J. Phys. Chem. C 117, 2245–2251 (2013)
Wang, J., Li, H., Ma, N.-N., Yan, L.-K., Su, Z.-M.: Theoretical studies on organoimido-substituted hexamolybdates dyes for dye-sensitized solar cells (DSSC). Dyes Pigments 99, 440–446 (2013)
Zhang, J., Li, H.-B., Zhang, J.-Z., Wu, Y., Geng, Y., Fu, Q., Su, Z.-M.: A promising anchor group for efficient organic dye sensitized solar cells with iodine-free redox shuttles: a theoretical evaluation. J. Mater. Chem. A 1, 14000–14007 (2013)
Zhang, Z.-L., Zou, L.-Y., Ren, A.-M., Liu, Y.-F., Feng, J.-K., Sun, C.-C.: Theoretical studies on the electronic structures and optical properties of star-shaped triazatruxene/heterofluorene co-polymers. Dyes Pigments 96, 349–363 (2013)
Mahmood, A., Tahir, M.H., Irfan, A., Al-Sehemi, A.G., Al-Assiri, M.S.: Heterocyclic azo dyes for dye sensitized solar cells: a quantum chemical study. Comput. Theor. Chem. 1066, 94–99 (2015)
Sun, L.L., Zhang, T., Wang, J., Li, H., Yan, L.K., Su, Z.M.: Exploring the influence of electron donating/withdrawing groups on hexamolybdate-based derivatives for efficient p-type dye-sensitized solar cells (DSSCs). RSC Adv. 5, 39821–39827 (2015)
Zhang, J., Li, H.-B., Sun, S.-L., Geng, Y., Wu, Y., Su, Z.-M.: Density functional theory characterization and design of high-performance diarylamine–fluorene dyes with different π spacers for dye-sensitized solar cells. J. Mater. Chem. 22, 568–576 (2012)
Li, M., Kou, L., Diao, L., Zhang, Q., Li, Z., Wu, Q., Lu, W., Pan, D., Wei, Z.: Theoretical study of WS-9-Based organic sensitizers for unusual vis/NIR absorption and highly efficient dye-sensitized solar cells. J. Phys. Chem. C 119, 9782–9790 (2015)
El Assyry, A., Jdaa, R., Benali, B., Addou, M., Zarrouk, A.: Optical and photovoltaic properties of new quinoxalin-2 (1H)-one-based DA organic dyes for efficient dye-sensitized solar cell using DFT. J. Mater. Environ. Sci. 6, 2612–2623 (2015)
Ait Aicha, Y., Bouzzine, S.M., Fahim, Z.M., Zair, T., Bouachrine, M., Hamidi, M.: Quantum chemical investigations study of the effect of electron donor units on the structural, electronic and optoelectronic properties of diarylthienopyrazine analogs. Comput. Theor. Chem. (2014). https://doi.org/10.1016/j.comptc.2014.03.008
Fahim, Z.M.E., Bouzzine, S.M., Ait Aicha, Y., Bouachrine, M., Hamidi, M.: The bridged effect on the geometric, optoelectronic and charge transfer properties of the triphenylamine–bithiophene-based dyes: a DFT study. Res. Chem. Intermed. (2017). https://doi.org/10.1007/s11164-017-3211-1
Gaussian09, R.A.: 1, MJ Frisch, GW Trucks, HB Schlegel, GE Scuseria, MA Robb, JR Cheeseman, G. Scalmani, V. Barone, B. Mennucci, GA Petersson et al., Gaussian. Inc., Wallingford CT (2009)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Krishnan, R., Binkley, J.S., Seeger, R., Pople, J.A.: Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 72, 650–654 (1980)
Fahim, Z.M.E., Bouzzine, S.M., Youssef, A.A., Bouachrine, M., Hamidi, M.: Ground state geometries, uv/vis absorption spectra and charge transfer properties of triphenylamine–thiophenes based dyes for DSSCs: a TD-DFT benchmark study. Comput. Theor. Chem. 1125, 39–48 (2018)
Becke, A.D.: A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993)
Barone, V., Cossi, M.: Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 102, 1995–2001 (1998)
Cossi, M., Rega, N., Scalmani, G., Barone, V.: Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 24, 669–681 (2003)
Frisch, A., Nielsen, A.B., Holder, A.J.: Gaussview user manual, p. 556. Gaussian Inc., Pittsburgh (2000)
Deschenes, L.A., Vanden, D.A.: BoutUniversity of Texas Austin: Origin 6.0: scientific data analysis and graphing software origin lab corporation (formerly Microcal Software, Inc.). www.originlab.com. Commercial price: 595. Academic price: 446, (2000)
Balanay, M.P., Kim, S.-M., Lee, M.-J., Lee, S.-H., Kim, D.-H.: Conformational analysis and electronic properties of 2-cyano-3-(thiophen-2-yl) acrylic acid in sensitizers for dye-sensitized solar cells: a theoretical study. Bull. Korean Chem. Soc. 30, 2077–2082 (2009)
Gianotti, V., Favaro, G., Bonandini, L., Palin, L., Croce, G., Boccaleri, E., Artuso, E., Van Beek, W., Barolo, C., Milanesio, M.: Rationalization of dye uptake on titania slides for dye-sensitized solar cells by a combined chemometric and structural approach. ChemSusChem 7, 3039–3052 (2014)
Bouzzine, S.M., Bouzakraoui, S., Bouachrine, M., Hamidi, M.: Density functional theory (B3LYP/6–31G*) study of oligothiophenes in their aromatic and polaronic states. J. Mol. Struct. Theochem. (2005). https://doi.org/10.1016/j.theochem.2005.04.023
El Mzioui, S., Bouzzine, S.M., Bouachrine, M., Bennan, M.N., Hamidi, M.: Effect of the alkyl chain length incorporated into donor part on the optoelectronic properties of the carbazole based dyes: theoretical study. Orbital (2017). https://doi.org/10.17807/orbital.v9i5.1003
Khan, S.U.-D., Mahmood, A., Rana, U.A., Haider, S.: Utilization of electron-deficient thiadiazole derivatives as π-spacer for the red shifting of absorption maxima of diarylamine–fluorene based dyes. Theor. Chem. Acc. 134, 1596 (2015)
Liu, J., Sun, X., Li, Z., Jin, B., Lai, G., Li, H., Wang, C., Shen, Y., Hua, J.: New D–π–A system dye based on dithienosilole and carbazole: synthesis, photo-electrochemical properties and dye-sensitized solar cell performance. J. Photochem. Photobiol. A 294, 54–61 (2014)
Huang, J.-F., Liu, J.-M., Tan, L.-L., Chen, Y.-F., Shen, Y., Xiao, L.-M., Kuang, D.-B., Su, C.-Y.: Novel carbazole based sensitizers for efficient dye-sensitized solar cells: role of the hexyl chain. Dyes Pigments 114, 18–23 (2015)
Geng, Y., Li, H.-B., Wu, S.-X., Su, Z.-M.: The interplay of intermolecular interactions, packing motifs and electron transport properties in perylene diimide related materials: a theoretical perspective. J. Mater. Chem. 22, 20840–20851 (2012)
Acknowledgements
The authors thank the Volubilis Program (No. Ma/11/248) for the purchase of Gaussian 09 and Mr. Abderrahmane Babni, Assistant Professor, Polydisciplinary Faculty of Errachidia Moulay Ismail University-Meknes, a teacher of English, who helped us to correct grammatical and spelling mistakes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Mzioui, S., Bouzzine, S.M., Bourass, M. et al. A theoretical investigation of the optoelectronic performance of some new carbazole dyes. J Comput Electron 18, 951–961 (2019). https://doi.org/10.1007/s10825-019-01339-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10825-019-01339-x