Abstract
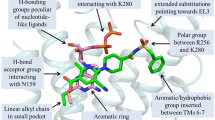
The P2Y12 receptor (P2Y12R) is an ADP-activated G protein-coupled receptor (GPCR) that is an important target for antithrombotic drugs. Three homology models of P2Y12R were compared, based on different GPCR structural templates: bovine rhodopsin (bRHO), human A2A adenosine receptor (A2AAR), and human C-X-C chemokine receptor type 4 (CXCR4). By criteria of sequence analysis (25.6% identity in transmembrane region), deviation from helicity in the second transmembrane helix (TM2), docked poses of ligands highlighting the role of key residues, accessibility of a conserved disulfide bridge that is reactive toward irreversibly-binding antagonists, and the presence of a shared disulfide bridge between the third extracellular loop (EL3) and the N-terminus, the CXCR4-based model appeared to be the most consistent with known characteristics of P2Y12R. The docked poses of agonist 2MeSADP and charged anthraquinone antagonist PSB-0739 in the binding pocket of P2Y12R-CXC agree with previously published site-directed mutagenesis studies of Arg256 and Lys280. A sulfonate at position 2 of the anthraquinone core created a strong interaction with the Lys174(EL2) side chain. The docking poses of the irreversibly-binding, active metabolite (existing as two diastereoisomers in vivo) of the clinically utilized antagonist Clopidogrel were compared. The free thiol group of the 4S diastereoisomer, but not the 4R isomer, was found in close proximity (~4.7 Å) to the sulfur atom of a disulfide bridge involving Cys175, suggesting greater activity in covalent binding. Therefore, ligand docking to the CXCR4-based model of the P2Y12R predicted poses of both reversibly and irreversibly-binding small molecules, consistent with observed pharmacology and mutagenesis studies.






Similar content being viewed by others
References
Dorsam RT, Kunapuli SP (2004) Central role of the P2Y12 receptor in platelet activation. J Clin Invest 113:340–345
Giossi A, Pezzini A, Del Zotto E, Volonghi I, Costa P, Ferrari D, Padovani A (2010) Advances in antiplatelet therapy for stroke prevention: the new P2Y12 antagonists. Curr Drug Targets 11:380–391
Jacobson KA, Deflorian F, Mishra S, Costanzi S (2011) Pharmacochemistry of the platelet purinergic receptors. Purinergic Signal. doi:10.1007/s11302-011-9216-0 (in press)
Moro S, Hoffmann C, Jacobson KA (1999) Role of the extracellular loops of G protein-coupled receptors in ligand recognition: a molecular modeling study of the human P2Y1 receptor. Biochemistry 38:3498–3507
Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745
Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF (2008) Structure of a beta1-adrenergic G-protein-coupled receptor. Nature 454:486–491
Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC (2007) High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 318:1258–1265
Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK (2007) Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450:383–387
Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, IJzerman AP, Stevens RC (2008) The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322:1211–1217
Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC (2010) Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330:1066–1071
Chien EY, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, Shi L, Newman AH, Javitch JA, Cherezov V, Stevens RC (2010) Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330:1091–1095
Scheerer P et al (2008) Crystal structure of opsin in its G-protein-interacting conformation. Nature 455(7212):497–502
Rasmussen SGF, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, DeVree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka KB (2011) Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469:175–180
Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC (2011) Structure of an agonist-bound human A2A adenosine receptor. Science. doi:10.1126/science.1202793 (in press)
Costanzi S, Joshi BV, Maddileti S, Mamedova L, Gonzalez-Moa MJ, Marquez VE, Harden TK, Jacobson KA (2005) Human P2Y6 receptor: molecular modeling leads to the rational design of a novel agonist based on a unique conformational preference. J Med Chem 48:8108–8111
Bissantz C, Bernard P, Hibert M, Rognan D (2003) Protein-based virtual screening of chemical databases. II. Are homology models of G-protein coupled receptors suitable targets? Proteins 50:5–25
Costanzi S, Mamedova L, Gao ZG, Jacobson KA (2004) Architecture of P2Y nucleotide receptors: structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem 47:5393–5404
Ballesteros J, Weinstein H (1995) Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci 25:366–428
Langelaan DN, Wieczorek M, Blouin C, Rainey JK (2010) Improved helix and kink characterization in membrane proteins allows evaluation of kink sequence predictors. J Chem Inf Model 50:2213–2220
Zhang R, Hurst DP, Barnett-Norris J, Reggio PH, Song ZH (2005) Cysteine 2.59(89) in the second transmembrane domain of human CB2 receptor is accessible within the ligand binding crevice: evidence for possible CB2 deviation from a rhodopsin template. Mol Pharmacol 68:69–83
Devillé J, Rey J, Chabbert M (2009) An indel in transmembrane helix 2 helps to trace the molecular evolution of class A G-protein-coupled receptors. J Mol Evol 68:475–489
Govaerts C, Blanpain C, Deupi X, Ballet S, Ballesteros JA, Wodak SJ, Vassart G, Pardo L, Parmentier M (2001) The TXP motif in the second transmembrane helix of CCR5. A structural determinant of chemokine-induced activation. J Biol Chem 276:13217–13225
Govaerts C, Bondue A, Springael JY, Olivella M, Deupi X, Le Poul E, Wodak SJ, Parmentier M, Pardo L, Blanpain C (2003) Activation of CCR5 by chemokines involves an aromatic cluster between transmembrane helices 2 and 3. J Biol Chem 278:1892–18903
Savi P, Zachayus J-L, Delesque-Touchard N, Labouret C, Hervè C, Uzabiaga M-F, Pereillo J-M, Culouscou J-M, Bono F, Ferrara P, Herbert J-M (2006) The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci USA 103:11069–11074
Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341
Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB (2001) Identification of the platelet ADP receptor targeted by anti-thrombotic drugs. Nature 409:202–207
Ivanov AA, Barak D, Jacobson KA (2009) Evaluation of homology modeling of G protein-coupled receptors in light of the A2A adenosine receptor crystallographic structure. J Med Chem 52:3284–3292
Hoffmann K, Baqi Y, Morena MS, Glänzel M, Müller CE, von Kügelgen I (2009) Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. J Pharmacol Exp Ther 331:648–655
Baqi Y, Atzler K, Köse M, Glänzel M, Müller CE (2009) High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y12 receptors. J Med Chem 52:3784–3793
Parlow JJ, Burney MW, Case BL, Girard TJ, Hall KA, Harris PK, Hiebsch RR, Huff RM, Lachance RM, Mischke DA, Rapp SR, Woerndle RS, Ennis MD (2010) Piperazinyl glutamate pyridines as potent orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation. J Med Chem 53:2010–2037
Hoffmann K, Sixel U, Di Pasquale F, von Kügelgen I (2008) Involvement of basic amino acid residues in transmembrane regions 6 and 7 in agonist and antagonist recognition of the human platelet P2Y12-receptor. Biochem Pharmacol 76:1201–1213
Collet JP, Montalescot G (2009) P2Y12 inhibitors: thienopyridines and direct oral inhibitors. Hämostaseologie 29:339–348
Algaier I, Jakubowski JA, Asai F, von Kügelgen I (2008) Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J Thromb Haemost 6:1908–1914
Rosenkilde MM, Benned-Jensen T, Frimurer TM, Schwartz TW (2010) The minor binding pocket: a major player in 7TM receptor activation. Trends Pharmacol Sci 31:567–574
Acknowledgments
We thank Dr. Stefano Costanzi (NIDDK) and Dr. David Erlinge (Lund University, Lund, Sweden) for helpful discussion. This research was supported by the Intramural Research Program of the NIH, NIDDK.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deflorian, F., Jacobson, K.A. Comparison of three GPCR structural templates for modeling of the P2Y12 nucleotide receptor. J Comput Aided Mol Des 25, 329–338 (2011). https://doi.org/10.1007/s10822-011-9423-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-011-9423-3