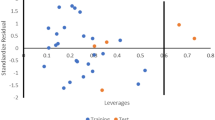
Abstract
Three-dimensional quantitative structure-activity relationship (3D QSAR) using comparative molecular field analysis (CoMFA) was performed on a series of substituted tetrahydropyran (THP) derivatives possessing serotonin (SERT) and norepinephrine (NET) transporter inhibitory activities. The study aimed to rationalize the potency of these inhibitors for SERT and NET as well as the observed selectivity differences for NET over SERT. The dataset consisted of 29 molecules, of which 23 molecules were used as the training set for deriving CoMFA models for SERT and NET uptake inhibitory activities. Superimpositions were performed using atom-based fitting and 3-point pharmacophore-based alignment. Two charge calculation methods, Gasteiger-Hückel and semiempirical PM3, were tried. Both alignment methods were analyzed in terms of their predictive abilities and produced comparable results with high internal and external predictivities. The models obtained using the 3-point pharmacophore-based alignment outperformed the models with atom-based fitting in terms of relevant statistics and interpretability of the generated contour maps. Steric fields dominated electrostatic fields in terms of contribution. The selectivity analysis (NET over SERT), though yielded models with good internal predictivity, showed very poor external test set predictions. The analysis was repeated with 24 molecules after systematically excluding so-called outliers (5 out of 29) from the model derivation process. The resulting CoMFA model using the atom-based fitting exhibited good statistics and was able to explain most of the selectivity (NET over SERT)-discriminating factors. The presence of −OH substituent on the THP ring was found to be one of the most important factors governing the NET selectivity over SERT. Thus, a 4-point NET-selective pharmacophore, after introducing this newly found H-bond donor/acceptor feature in addition to the initial 3-point pharmacophore, was proposed.










Similar content being viewed by others
References
Amara SG, Kuhar MJ (1993) Annu Rev Neurosci 16:73
Laakso A, Hietala J (2000) Curr Pharm Des 6:1611
Hahn MK, Blakely RD (2002) Pharmacogenomics J 2:217
Heinz A, Mann K, Weinberger DR, Goldman D (2001) Alcohol Clin Exp Res 25:487
Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA (1997) J Neurosci 17:8451
Robertson D, Flattem N, Tellioglu T, Carson R, Garland E, Shannon JR, Jordan J, Jacob G, Blakely RD, Biaggioni I (2001) Ann N Y Acad Sci 940:527
Miller GM, De La Garza R II, Novak MA, Madras BK (2001) Mol Psychiatry 6:50
Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL (2003) Mol Psychiatry 8:933
Nemeroff CB, Owens MJ (2002) Nat Neurosci 5:1068
Dutta AK, Zhang S, Kolhatkar R, Reith MEA (2003) Eur J Pharmacol 479:93
Olivier B, Soudijn W, van Wijngaarden I (2000) Prog Drug Res 54:59
Walter MW (2005) Drug Develop Res 65:97
Miller DK, Wong EHF, Chesnut MD, Dwoskin LP (2002) J Pharmacol Exp Ther 302:687
Scates AC, Doraiswamy PM (2000) Ann Pharmacother 34:1302
Hajos M, Fleishaker JC, Filipiak-Reisner JK, Brown MT, Wong EHF (2004) CNS Drug Rev 10:23
Koch S, Hemrick-Luecke SK, Thompson LK, Evans DC, Threlkeld PG, Nelson DL, Perry KW, Bymaster FP (2003) Neuropharmacology 45:935
Thase ME, Entsuah AR, Rudolph RL (2001) Br J Psychiatry 178:234
Zhang S, Reith MEA, Dutta AK (2003) Bioorg Med Chem Lett 13:591
Zhang S, Zhen J, Reith MEA, Dutta AK (2004) Bioorg Med Chem 12:6301
Zhang S, Zhen J, Reith MEA, Dutta AK (2005) J Med Chem 48:4962
Zhang S, Fernandez F, Hazeldine S, Deschamps J, Zhen J, Reith MEA, Dutta AK (2006) J Med Chem 49:4239
Cramer RD III, Patterson DE, Bunce JD (1988) J Am Chem Soc 110:5959
Vogel AI (1989) Vogel’s textbook of practical organic chemistry, 5th edn. Longman, Harlow
SYBYL Molecular Modeling System, Version 7.1, Tripos Inc., St. Louis, MO 63144–2913
Wong G, Koehler KF, Skolnick P, Gu ZQ, Ananthan S Schonholzer P, Hunkeler W, Zhang W, Cook JM (1993) J Med Chem 36:1820
Matter H, Schwab W (1999) J Med Chem 42:4506
Böhm M, Stürzebecher J, Klebe G (1999) J Med Chem 42:458
Clark M, Cramer RD III, Van Opdenbosh N (1989) J Comput Chem 10:982
Stewart JJP (1990) J Comput-Aided Mol Des 4:1
Dunn WJ, Wold S, Edelund U, Helberg S (1984) Quant Struct-Act Relat 3:131
Cramer RD III, Bunce J, Patterson DE (1988) Quant Struct-Act Relat 7:18
van de Waterbeemd H, Testa B, Folkers G (eds) (1997) Computer-Assisted Lead Finding and Optimization, Verlag Helvetica Chimica Acta (VHCA). Basel and Wiley–VHC, Weinheim, pp 445–459
Acknowledgements
This work was supported by the National Institute on Drug Abuse, Grant No. DA 12449 (AKD). We acknowledge Stuart Hazeldine for his help with the synthesis and Juan Zhen for her help with the biological characterization of compounds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kharkar, P.S., Reith, M.E.A. & Dutta, A.K. Three-dimensional quantitative structure-activity relationship (3D QSAR) and pharmacophore elucidation of tetrahydropyran derivatives as serotonin and norepinephrine transporter inhibitors. J Comput Aided Mol Des 22, 1–17 (2008). https://doi.org/10.1007/s10822-007-9146-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-007-9146-7