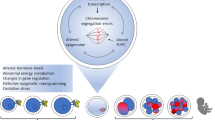
Abstract
In mammalian species an optimal fertilization window during which successful fertilization occurs. In the majority of mammals estrus marks ovulation time and coincident with mating, thereby allowing the synchronized meeting in the fallopian tubes, between freshly ejaculated sperm and freshly ovulated oocytes. Conversely, women do not show natural visual signs of ovulation such that fertilization can occur hours later involving an aged oocyte and freshly ejaculated spermatozoa. During this time, the oocyte undergoes a rapid degradation known as “postovulatory aging” (POA). POA may become particularly important in the human-assisted reproductive technologies, as the fertilization of retrieved mature oocytes can be delayed due to increased laboratory workload or because of unforeseeable circumstances, like the delayed availability of semen samples. This paper is an updated review of the consequences of POA, either in vivo or in vitro, on oocyte quality with particular attention to modifications caused by POA on oocyte nuclear, cytoplasmic, genomic, and epigenetic maturation, and embryo development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mature oocytes arrested at meiotic metaphase II (MII) can be successfully fertilized if the insemination occurs in a restricted time window after ovulation, within 12 h for rodents and 24 h for monkeys and humans. Otherwise, oocytes undergo a time-dependent process of degradation referred to as “postovulatory aging” (POA). In comparison with oocytes analyzed soon after ovulation, these aged oocytes evidence several morphological, molecular, genomic, and epigenetic anomalies [1,2,3,4,5,6] that can even result in apoptosis [1, 2, 7]. In humans, oocytes retrieved during the assisted reproduction technology (ART) procedures can be subjected to extended periods of culture prior to fertilization, thus reducing the viability of the resulting embryos [3]. Both in vivo and POA have been associated with reduced fertilization rates, poor embryo quality, implantation failure, and abnormalities in the offspring [3].
In contrast with the physiological fertilization [8, 9], the common effects of POA in oocytes are the degeneration of polar body (PB) I, the incomplete extrusion of PBII associated with disruption of meiotic spindle [10], increased perivitelline space and partial cortical granules (CGs) exocytosis [11]. Other morphological modifications such as shrinkage, membrane blebbing, cytoplasmic fragmentation and granulation, and degeneration [9, 12] are mainly caused by increased reactive oxygen species (ROS) production.
The current review will focus only on POA-related mechanisms (Fig. 1) that we kept separately from preovulatory aging. The latter involves the female age-related damage of oocytes that cumulates along the advanced maternal age. Although the biological consequences of post and preovulatory oocyte aging can largely overlap and will elevate the risks for fertilization failure, compromised embryo viability, miscarriage, and offspring diseases, the molecular mechanism of both types of oocyte aging can be uniquely distinguished.
Main POA-related morphological and molecular alterations in mammalian oocytes. After ovulation of the MII-stage oocyte, POA-related mechanisms occur in unfertilized oocytes. Morphological alterations include ZP hardening, mt and CGs abnormal distribution patterns, CGs vacuolization, and spindle organization. On a molecular level, POA induces the increase of intracellular ROS levels and oxidative stress mechanisms, inducing lipids peroxidation, mt and DNA oxidative damages (arrows), and Ca2+ release from ER. Oxidative stress and cytochrome C release from mt activate apoptotic mechanisms that, together with higher intracellular Ca2+ levels, inactivate the cell cycle regulators (MPF, MAPK) and destabilize the epigenetic pattern (i.e., histone acetylation). Abbreviations: ATP, adenosine triphosphate; Ca2+, calcium; cAMP, cyclic adenosine monophosphate; Cas 3, caspase 3; CGs, cortical granules; Cyt C, cytochrome C; ER, endoplasmic reticulum; MAPK, mitogen-activated protein kinase, MII: metaphase II; MPF, maturation promoting factor; mt, mitochondria; POA, postovulatory aging; ROS, reactive oxygen species; ZP, zona pellucida
A brief overview of in vivo and in vitro oocyte meiotic maturation
Since POA can occur either in vivo or in vitro, the time-dependent degradation/inactivation of proteins involved in nuclear and cytoplasmic remodeling plays a key role in fertilization failure and disrupted embryo viability.
It is universally accepted that the production of a fertilizable oocyte requires the coordinate development of both germ and somatic granulosa cells (GCs) from primordial up to preovulatory stage [5]. In terminally differentiated follicle, the fully grown oocyte, still arrested at diplotene of prophase I (germinal vesicle, GV), is closely surrounded by several thousands of specialized GCs, the cumulus cells (CCs); the other GCs, the mural granulosa cells, line the follicular wall and define the fluid-filled antral cavity [4, 5]. The main feature of oocyte meiotic maturation is the achievement of an MII-arrested haploid oocyte, and this process is regulated by similar molecules in animals as in humans [13].
Oocytes are arrested at GV stage until the luteinizing hormone (LH) surge. This arrest is maintained by adequate levels of cyclic adenosine monophosphate (cAMP) that are finely regulated by a balanced mechanism of synthesis and degradation [6]. Surrounding GCs transmit to the oocyte signals involved in the regulation of intraoocyte cAMP levels. This regulation is based on a regulative loop [14], stimulated not only by follicle stimulating hormone (FSH) but also by sex hormones, as androgen and estrogen [15, 16].
The midcycle surge of LH determines several irreversible effects [17], including activation of the epidermal growth factor (EGF), EGF/EGFR pathway [18]. EGFR signaling in turn regulates CCs expansion and coordinates oocyte cytoplasmic maturation, thereby influencing the developmental capacity of the oocyte [19, 20].
The activation of the maturation promoting factor (MPF) triggers the germinal vesicle breakdown (GVBD) and chromosome segregation via securin degradation [21, 22]. Following GVBD, meiosis I is completed by discharging the PBI with a haploid set of chromosomes; subsequently, the oocytes enter meiosis II but arrest at MII stage until fertilization because of a high MPF activity [23]. This resting state can be stable for many hours and is dependent on a cytoplasmic activity termed CSF, which inhibits the anaphase-promoting complex/cyclosome (APC/C). Once the oocyte will be fertilized, APC/C will become active and cyclin B degraded. Members of the Emi/Erp family of proteins are components of CSF: Emi2, which is controlled by Mos-mitogen-activated protein kinase (MAPK) pathway, competitively inhibits APC/C binding before fertilization, while its degradation after fertilization reduces its inhibitory action [24, 25].
Along with nuclear maturation, cytoplasmic maturation of oocytes implies the synthesis, activation, and degradation of maternal mRNA, as well the rearrangement of organelles, especially CGs, mitochondria (mt), endoplasmic reticulum (ER), and cytoskeleton (for more details: [26]). Usually, a low oocyte quality correlates with abnormal mt and ER rearrangement. In fact, alterations of adenosine triphosphate (ATP) production of free Ca2+ storage/release in the oocyte cytoplasm as well as of microtubules/microfilament dynamics compromise irreversibly fertilization process [27].
In vitro maturation (IVM) is a technology by which immature oocytes retrieved from antral follicles can be maintained in a suitable culture medium until reaching the MII stage. This definition however is not sufficient for human IVM, since the stimulation protocols, follicle size, and time of oocyte retrieval influence its outcome. In humans, IVM is considered restricted to a limited number of patients such as those with polycystic ovary syndrome, history of ovarian hyperstimulation syndrome or poor responders. During ART procedures, some GV and metaphase I (MI) oocytes can be retrieved from antral follicles. The finding that the in vitro culture of immature oocytes seems to improve more nuclear than cytoplasmic maturation [28] suggests that these events can occur simultaneously but independently of each other. Although the comparison of in vivo and in vitro matured human oocytes reveals many morphological similarities [29,30,31], extending culture period can lead to POA and increased risk of aneuploidies.
Postovulatory aging in vivo studies
Delayed fertilization of ovulated oocytes leads to the activation of a plethora of biochemical and molecular mechanisms that impairs oocyte quality and jeopardizes the success of embryo formation and development [32].
In order to better understand the mechanisms underlying this phenomenon in vivo, studies have been conducted using animal models, particularly mouse and rat, by retrieving the ovulated oocytes after several hours’ post-human chorionic gonadotropin (hCG) injection. In this section, we report the main findings present in literature.
Morphological alterations
During the past years, several in vivo studies demonstrated interesting findings on the effect of POA on the fine oocyte structure, which manifest as a variety of morphological alterations that impact reproductive outcome. Postovulatory changes occur in a short time. CGs distribution is altered, because some areas appeared to be free of CGs, which are in part vacuolated, in part internalized into the ooplasm, and in part swollen [33]. Numerous dense structures, vesicles (lysosome-like vesicles) are present, and ooplasmic fragmentation is often observed. This could be related to the apoptotic phenomenon described in aged mouse oocytes [33]. Similar changes have been found in mouse oocytes 24 h after ovulation: the percentage of swollen CGs that are internalized from the membrane into the central region of the oocyte increases, as they lose their anchorage in the cortex [34, 35]. In mice, in vivo administration of caffeine, a potent antioxidant, causes, on the one hand, a significant decrease in the normal oocyte percentage, and, on the other hand, premature CGs exocytosis in low-quality oocytes, and a delay in exocytosis and congregation of CGs in good-quality oocytes [36]. In addition, the decreased numbers of CGs, together with a time-dependent increase in CGs exocytosis, as well as zona pellucida (ZP) hardening, have been observed in mouse POA-oocytes [37,38,39]. In vivo short-term resveratrol treatment has no impact on mt distribution, nor in preventing spindle aberrations in mouse POA-oocytes. In fact, Liang and coworkers found that microtubules displayed two distribution patterns, a homogeneous and a clustered one, in both resveratrol-treated and control groups. Also, they detected the presence of a variety of altered spindles as abnormal, elongated, without poles, mono-polar, multi-polar, and unorganized spindles with astral microtubules, together with the presence of many ooplasmic asters [40]. Similarly, Wortzman and Evans described microtubule aster-like bundles in the ooplasm and in the cortex of POA-oocytes in mice [41]. A 25-h-long aging causes altered structure of ER and mt network, facilitating formation of the cistern aggregates [42]. Also, chromosomal abnormalities have been reported in unfertilized in vivo POA-oocytes [43], and the presence of distended amicrovillar domains has been evidenced by after 21–22-h post-hCG [44]. Interestingly, Dalo and collaborators in mouse POA oocytes described three different phenotypes of microvillar and amicrovillar domains that are all equally correlated with the impaired ability of being fertilized [45]. In particular, the first phenotype shows a normal microvillar surface, increased fertilization cones with wrinkled appearance; the second shows a microvillar region where occasionally are present in bulb-like vesicles and small distinct pits, vesicles related to the precocious exocytosis and CGs loss [37]; the third one is with small amicrovillar patches over the site of sperm incorporation.
Oxidative stress, ATP, and mitochondrial membrane potential
cAMP is universally recognized as the key regulator of GV oocytes meiotic maturation arrest. Nonetheless, the cyclic nucleotide, ATP, and AMP-activated protein kinase (AMPK) are involved in MII-stage oocyte arrest before fertilization [9, 46]. After ovulation, the MII-stage oocyte is released in the fallopian tubes, thus being subjected to different environmental stimuli, as the oxidative stress (OxS) that, if prolonged in time, activate several biochemical and molecular processes intended for egg activation [47, 48].
Igarashi and collaborators investigated modifications in intracellular ATP concentration in fresh and in vivo mouse POA-oocytes [49]. The authors suggested that aged oocytes exposed to increased level of OxS undergo a dysregulation of mt ATP production that produces an incomplete readjustment of ATP levels at fertilization. ATP dysregulation could directly or indirectly (via altered Ca2+ oscillations) affect the oocyte quality and, after fertilization, the embryo development [49]. To further confirm mt role in POA-oocytes, the same research group investigated mt membrane potential (MMP), oxygen consumption rate and mitochondrial transcriptional factor A (TFAM) [50]. Results showed that in POA-oocytes, both MMP and oxygen consumption rates were significantly lower compared to fresh oocytes. Taken together, the mt instability results in incomplete readjustment of ATP levels, leading to an impaired intracellular Ca2+ homeostasis and to a poor embryonic development [50]. On the other hand, while the total TFAM expression was similar between fresh and POA-oocytes, its colocalization with mt decreased with time, suggesting an impaired mt biogenesis. It is noteworthy that attempts to rescue oocyte fertilization quality by microinjecting mt from other somatic cell types into aged oocytes failed in both mice [50] and women [51].
The previous results are further confirmed by a recent study from Sun and collaborators that reported a significant decrease (almost 40%) in cAMP levels in mouse oocytes collected from the fallopian tubes after 24 h from ovulation [46]. The decline in cAMP, followed by inactivation of protein kinase A, promoted AMPK activation that facilitated oocyte aging through the inhibition of MPF downstream signaling via increasing ROS.
These data are connected directly and indirectly to the presence or the release of ROS in POA-oocytes, where a gradual accumulation of hydrogen peroxide, super-oxide anion, and peroxynitrite reactive compounds occurs [3, 11]. Sirtuin (SIRT) family genes are highly relevant to OxS, since their modulation is correlated with ROS presence, and the expression of these genes helps the recovery of a more stabilized cellular environment [52,53,54]. Zhang and collaborators demonstrated that oocyte mRNA levels of Sirt1, 2, and 3 significantly decreased in a time-dependent manner [55]. In mice, literature data corroborate that short-term injections of resveratrol, a natural phenolic compound/phytoalexin, effectively ameliorated OxS-induced damage by increasing the mRNA and protein expression of SIRT1, reducing the ROS intracellular levels and improving mt function [40]. If not stabilized by the antioxidant machinery of the cell, the increasing level of ROS in the cytoplasm often results in damaged cellular and mt proteins, lipids, and DNA, one of the major OxS targets [3].
Intracellular Ca2+ homeostasis
As mentioned above, OxS is responsible for the activation of several molecules and factors that subsequently alter downstream biochemical and molecular mechanisms. To this, both OxS and ATP/cAMP dysregulation result in impaired Ca2+ homeostasis [52], linked to ER stress-related environment [56].
In rat, Premkumar and Chaube demonstrated that an insufficient increase in free intracellular Ca2+ induces POA-derived abortive spontaneous egg activation (SEA) in a time-dependent fashion (~ 60% after 17-h post-hCG injection, and 100% after 19 h) [8]. The importance of intracellular Ca2+ level is corroborated by recent studies that found abnormal Ca2+ oscillations in mouse POA-oocytes compared to fresh ovulated oocytes [57, 58], correlating it to higher fragmentation and altered mt membrane permeability which leads to the release of proapoptotic factors [57]. Specifically, the source of Ca2+-impaired homeostasis is linked to ER-specific ATPase activity suppression, depletion of Ca2+ levels in ER [59], and inositol 1,4,5-trisphosphate (IP3)-dependent mechanisms misfunction [60]. These processes are due to ER stress environment, as demonstrated by Takehara and collaborators that noticed a significant increase in Grp78, an ER stress-related marker [56].
Overall, the unbalanced Ca2+ levels caused by a prolonged retaining period in the oviducts may interfere with later processes crucial for sperm fertilization in mice [61] and with parthenogenetic activation and male pronuclei formation after ICSI in golden hamsters [62].
Cell cycle regulatory factors and spindle-associated proteins
One of the mechanisms affected by delayed fertilization is cell cycle regulation, mainly via the downregulation of MPF and MAPK proteins [63]. As direct or indirect consequence of both cAMP and Ca2+ altered levels, MPF inactivation induces oocyte activation. On the one hand, cAMP-dependent AMPK activation gradually inactivates MPF in a timely manner (from 13 to 24 h after hCG injection) [46]. On the other hand, higher levels of Ca2+ activates calmodulin-dependent protein kinases that destabilize MPF through the APC/C activation of MPF regulator, Wee1. This kinase triggers the dissociation of cyclin B1 from cyclin-dependent kinase 1 (Cdk1), leading to its ubiquitination and degradation and PBII extrusion [9, 46].
Mimicking MPF trend, MAPK1/3 reduced activity is demonstrated in both mouse [39] and cat oocytes [64]. In in vivo mouse POA-oocytes, kinase levels undergo a significant decrease, affecting the phosphorylation of the myosin-II regulatory light chain and increasing the rates of parthenogenetic activation [44].
As MPF and MAPK, the spindle-associated kinase Akt1/2/3 is highly deregulated during POA, together with the translocation of γ-tubulin from spindle poles to microtubules [65, 66]. Specifically, the mRNAs of all the isoforms are drastically reduced during in vivo POA, and the phosphorylated forms (Ser473- and Thr308-pAkt), generally associated with spindle poles disappear during POA in a time-dependent fashion [65]. Moreover, the absence of actin-rich cap or the presence of an abnormal actin-rich protrusion over the meiotic spindle has been observed in mouse POA oocytes after 24–48-h post-hCG [41]. Finally, also the reduced expression of Mad2 transcript found after 19- and 24-h post-hCG can contribute to abnormal spindle functionality in the process of chromosomal segregation [67], together with the translationally controlled tumor protein, which has been shown to be necessary for spindle assembly dynamics in oocytes during POA [68]. Taken together, these results suggest a propension of the POA-oocyte to meiotic errors and unfavorable conditions for either fertilization or successful embryo development.
Apoptosis
DNA fragmentation, mt dysfunction, and impaired Ca2+ homeostasis caused by high OxS often result in the release of pro-apoptotic factors (e.g., cytochrome c), and the dysregulation of anti-apoptotic factors (e.g., BCL2) has been frequently observed in in vivo POA-oocytes [11, 42, 69]. Transcriptional control of anti- and pro-apoptotic markers has been also linked to the differential expression of apoptosis-related microRNAs (miRNAs) during in vivo POA [70]. In their study, Wang and collaborators highlighted the presence of 6 upregulated miRNAs related to apoptosis, of which one promotes anti-apoptotic mechanisms and 5 promote pro-apoptotic mechanisms, as the upregulation of caspase 3 through miR-98 [70]. The increased activity of caspase 3 leads to increased intracellular Ca2+ levels and, subsequently, increased STAS levels. Moreover, the increment in miR-15a and miR-16 copies could be related to a post-transcriptional regulation of BCL2 [71].
Postovulatory aging in vitro studies
To deepen the knowledge in POA-inducing mechanisms and to test the effectiveness of “antiaging” molecules, in vitro studies have been conducted in humans and animal models, among which rat, mouse, and pig, by retrieving the ovulated MII-stage oocytes and culturing them in the presence or absence of antioxidant molecules and other natural/chemical additive to modulate POA (Table 1). Timings of in vitro experimental studies included in this review are summarized in Fig. 2. In this section, we report the main findings present in literature that suggest the use of this “antiaging” molecules as a potential tool against in vitro POA during ART procedures.
Timeline of meiotic maturation and POA in in vitro studies in different mammals: human, mouse, rat, and pig. Schematic summary of the average different timings utilized for meiotic maturation and activation of POA in the in vitro studies reported in this review. For humans, see [13]. Abbreviations: GV, germinal vesicle; MI, metaphase I; MII, metaphase II; POA, postovulatory aging
Morphological alterations
In vitro POA seems to have a greater impact on oocyte morphology and many studies focus on the caused organelle alterations of oocytes that may limit the reproductive outcome. In mice, in vitro POA-oocytes surrounded by CCs showed a partial CGs exocytosis and ZP hardening, while very few CC-free oocytes released their CGs, suggesting that CCs may have a role in fastening POA processes in vitro [39]. Additionally, caffeine supplementation caused an acceleration of the abnormal CGs distribution and an increase of CGs exocytosis [36], in accordance with the in vivo findings reported above. Morphological observations in mouse aged oocytes revealed aggregates of ER cisterns and of mt [42], similar to those reported in vivo, and an increase in the abundance of large autophagic lysosomes and in spindle length [89]. After 24 and 48 h, a defective spindle assembly and a loss of mt normal distribution — completely or partially — and of normal CGs localization in the subcortex have been detected in porcine oocytes [90]. Also in in vitro mouse POA-oocytes, increased frequency of spindle defects and abnormal distribution of mt pattern have been found after treatment with nicotinamide (NAM), indicating that inhibition of sirtuin family members (SIRT1, 2, 3) may accelerate degenerative processes [55]. Mouse oocytes cultured for 12 h, 18 h, and 24 h showed increased percentages of abnormal spindles, mt distribution, and CGs distribution, and the treatment with N-acetyl-L-cysteine (NAC) antioxidant helped in alleviating these modifications [79]. Similar findings regarding the improvement of abnormal CGs distribution have been described when melatonin was added in vitro during oocyte culture [91]. However, even if resveratrol treatment had a beneficial effect on spindle integrity, morphology, and chromosomal alignment in in vitro mouse POA-oocytes, as well as on mt distribution, the CGs distribution was still found to be disturbed [92, 93]. Coenzyme Q10 (CoQ10) supplementation in mouse POA-oocytes aged for 24 h in vitro recovered spindle assembly, misalignment of chromosome, abnormal distribution patterns of mt, and CGs distribution (discontinued or completely disappeared signals of CGs) [94]. Jia and collaborators also found abnormal spindles, as condensation, elongation, dispersal, or disruption occurred and chromosome failed to align at the metaphase plate in in vitro porcine POA-oocytes [95], but astaxanthin supplement showed to maintain spindle organization and the functional status of mt, ER, Golgi apparatus, and lysosomes. Ubiquinol-10 rescued aging-induced cytoskeleton impairment and even if POA caused an increase in abnormal spindles, ubiquinol-10 decreased the rate of abnormal spindles at both 24 and 48 h of aging, in pigs [96]. Oocyte morphological defect and increased mt aggregation have been found in mouse oocytes aged 24 h in vitro [72]. However, dose-dependent melatonin treatments partially restored these morphological defects [72]. Oocytes aged for 24 h in vitro mostly showed normal morphology with low fragmentation, while after 48 h, their ooplasm was non-uniform and it was dispersed being dark pigmented [75]. More than half of the 48-h porcine POA-oocytes became fragmented, but melatonin treatment improved the abnormal morphology and contributed to decrease fragmentation percentage [75]. Disruption or loss of the microfilament domain underlying the plasma membrane was found in in vitro porcine POA-oocytes [77]. Notably, a study on human POA-oocytes showed altered ultrastructure of ZP and of organelles, as evidenced by a reduction of mt-smooth ER aggregates size and amount, an increase of mt-vesicle complexes size and amount, a decrease of CGs and microvilli, and alterations of the spindle structure [78]. However, quercetin, icariin, and Artemisia asiatica treatments attenuated the aging-associated morphological abnormalities [80,81,82]. Notably, Ogawa and coworkers demonstrated an improvement of the developmental competence of in vitro POA-oocytes through the MII spindle transfer in mice [97].
Oxidative stress, ATP, and mitochondrial membrane potential
In vitro studies in different animal models showed an increase in ROS production during POA, as demonstrated in porcine [75, 76, 83, 90, 95, 96, 98, 99] and murine species [46, 55, 72, 79,80,81, 84, 91, 93, 100]. In mice, supplementation of NAC antioxidant used at 0.6 mM showed a decrease in ROS production and further OxS-related mechanisms during in vitro POA after 18–24 h, followed by an increase of ATP levels [79, 85]. Also in porcine oocytes, Niu and collaborators evidenced the protective effects of ubiquinol-10 against ROS increment and ATP/mt activity depletion [96]. Furthermore, the authors found an improved mt biogenesis, through the measurement of mRNA expression of mt genes as SIRT1 and PGC1A in ubiquinol-10-treated POA-oocytes [96].
Melatonin treatment in vitro appears to relieve in a time-dependent manner the initial OxS process activation [1]. In mouse [91] and porcine [75] oocytes, literature data demonstrated that melatonin-supplemented culture helps in significantly decreasing ROS levels, along with increasing the transcription of anti-OxS gene GPX4 and mt-related gene POLG2, and the recovery in MMP. On the contrary, melatonin treatments are not enough for increasing intracellular glutathione levels nor the transcription of anti-oxidative and mt related genes, as SOD1, CAT, CYCS, SIRT1, and AKT2 [75]. Similar results are obtained when oocytes are cultured in the presence of resveratrol (mouse [93], pig [76]), extracts of Artemisia asiatica [81], astaxanthin [95], bezafibrate (Bez) [98], CoQ10 [94], imperatorin [101], and icariin [82].
The different levels in cytoplasmic ROS may also be connected to the protected role of SIRT proteins, as demonstrated in mouse POA-oocytes treated with the SIRT1/2/3 inhibitor, NAM. After 6 and 12 h of exposure to 5 mM NAM, a SIRT-dependent increase in ROS levels is detected in comparison with control oocytes [55]. On the other hand, treatment with caffein 10 mM delayed the POA process by stimulating the transcription of SIRTs [55]. The important role of SIRTs has been proved also in mouse POA-oocytes treated with quercetin [80], that showed a dose-dependent preventive action in Sirt-1, -2, and -3 transcriptional levels decrease, leading to reduced ROS accumulation and spindle abnormalities. Similarly, in porcine POA-oocytes treated with putrescine, Xu and collaborators reported intracellular ROS decrease, and SOD2, SIRT1, and FOXO3 mRNA and protein expression level rescue, together with an increased MMP index [83]. Notably, the dysfunction provoked by an abnormal MMP index, and its connection to ROS, AMPK,and cAMP levels, has been proved in mouse POA-oocytes treated with metformin [46]. In brief, metformin induces the production of ROS after the activation of AMPK, leading to a decline in cAMP intracellular levels, destabilizing the MMP and facilitating POA mechanisms [46]. In addition, the imperatorin-supplemented culture of porcine oocytes enhance the MMP, oxidation resistance through enzymatic activation of SOD and CAT, and GSH levels in porcine POA-oocytes [101].
Intracellular Ca2+ homeostasis
As POA-related mechanisms can induce abortive SEA, Premkumar and Chaube demonstrated the importance of intracellular Ca2+ balance and oscillations by culturing rat oocytes in the presence or absence of the Ca2+ channel blocker nifedipine. The results show how a partial increase in Ca2+ levels (promoted by nifedipine) can induce abortive SEA, while a further increase (induced by Ca2+ supplementation) can induce physiological egg activation [8]. In both mouse and pig oocytes, literature data link the alteration in Ca2+ oscillation to its depletion from ER stores and, subsequently, release of proapoptotic factors triggered by increased MMP, resulting in POA-oocytes fragmentation [42, 99]. POA-related ER stress and dysfunction induce biochemical modification of IP3R1, thus impairing the correct release of Ca2+ from ER that can be prevented by caffeine in vitro treatment [60].
Notably, using the Kinex™ KAM-850 Antibody Microarray for the detection of protein kinases, phosphatases, and related activators/regulators, McGinnis and collaborators found that Ca2+/calmodulin-dependent protein kinases (CAMKs) family members were overexpressed (> 30%) in mouse POA-oocytes after just 8 h of culture [89]. This result, together with the decreased expression of Ca2+ binding proteins, calnexin, and calreticulin, shows an impairment in Ca2+ homeostasis [89]. Moreover, Sun and collaborators found that the inhibition of CAMKs through STO-609 together with oocyte metformin-treatment increased levels of active AMPK during POA processes, suggesting a link between AMPK, Ca2+ signaling and CAMKs [46].
Cell cycle regulatory factors and spindle-associated proteins
Alike with in vivo POA studies, when MII-ovulated oocytes are cultured in vitro, the cell cycle factors are subjected to deregulation, thus accelerating degenerative mechanisms. In rat, mouse, and porcine oocytes, in vitro POA increased Cdk1 Thr14/Tyr15 phosphorylation and activity and decreased cyclin B1 levels, thus destabilizing MPF activity [46, 80, 102, 103]. The use of RO-3306, a Cdk1 inhibitor, prevents Cdk1 phosphorylation and further cyclin B1 ubiquitination, providing stability for MPF and delaying POA [102]. The deregulation of MPF is generally featured by an early MAPK inactivation [83, 103, 104] and cell cycle-related kinases and phosphatases, as Chek1 and 2, Cdc25c [89]. These degenerating processes can be delayed by supplementing culture medium with antioxidants (e.g., quercetin) and polyamines (e.g., putrescine) that act by downregulating Cdk1 Tyr15 phosphorylation and MAPK1/3 inhibition, and upregulating cyclin B [80, 83].
Interestingly, McGinnis and coworkers found an extensive deregulation of spindle-associated proteins and kinases involved in spindle assembly and stability, as SRC-family proteins, ABL, and the aurora kinase A, B, and C [89]. In mouse oocytes, in vitro POA mechanisms are responsible for time-dependent downregulation of MAD2 [10, 104], and the formation of the actin cap over the spindle [91], causing destabilization in the spindle formation and errors in sister chromatin segregation. The supplementation of melatonin in cultured oocytes can reduce the rate of aberration in actin filaments formation and spindle-related abnormalities [91].
Apoptosis
After the first hours of in vitro oocyte culture (both murine and porcine), the occurrence of POA-related OxS coincides with the activation of early apoptosis mechanisms, including phosphatidylserine externalization and BCL2 downregulation [1, 76, 80, 81, 89, 90, 93, 103, 104]. The activation of cell death mechanisms can be prevented by a treatment of in vitro POA-oocytes with melatonin [1] that inhibits the decrease of expression of BCL2 and the overexpression of pro-apoptotic genes Bax and Bad [80], as well as with putrescine [83], extracts of Artemisia asiatica [81], quercetin [80], resveratrol [76, 93], and imperatorin [101].
The use of antiaging molecules resulted often also in a decreased detection of caspases in POA-oocytes, suggesting a protective role of these supplements during oocyte culture. Particularly, caspase 3 downregulation has been proved in the presence of resveratrol [76], quercetin [80], and Bez [98]. Putrescine-treated oocytes display a recovery in caspase 3 and caspase 9 activation that were significantly reduced after 24 h of culture in porcine oocytes [83].
Notably, recent literature data demonstrated the activation also of autophagy markers in in vitro POA-oocytes [73]. In particular, the supplementation with ubiquitinol-10 and imperatorin appeared to prevent the overexpression of apoptosis- and autophagy-related genes, as CASP3, SURVIVIN, ATG5, ATG7, and LAMP2, after 24 h of culture [96, 101].
Epigenomic and genomic aberrations in postovulatory aging
In the last decades, various groups have evidenced that POA mechanisms can interfere with the normal epigenetic asset before and after fertilization, thus affecting embryo epigenome and its further development [74, 86]. In vivo and in vitro studies examined DNA methylation among the epigenetic modifications and found a POA-dependent loss of methylation in the CpG of maternal imprinting genes Snrpn and Peg1/Mest after 13 h of culture and 29-h post-hCG injection [87, 88]. Furthermore, studies in mouse POA-oocytes detected a significant aging-dependent increase in pericentromeric ATP-dependent helicase (ATRX) [105], together with abundance in histone H4K12 acetylation and pericentromeric histone H3K9 demethylation [93, 105]. These findings are in accordance with studies in mice and pigs reporting increased histone acetylation also in H3K14 and H4K8 [64, 65, 106,107,108]. On the contrary, Huang and coworkers showed an accelerated aging process when mouse oocytes treated for 5 h with trichostatin A, due to raising levels of histone acetylation [106].
A study from Dankert and collaborators investigated transcript levels and their posttranscriptional poly(A) tail length of maternal effect genes [109]. The quantitative RT-PCR analysis revealed a significant shortening in poly(A) tails of Nlrp5, Tet3, Trim28, and Dnmt1, after both in vivo and in vitro POA [110]. These results, together with the chromatin and histone modification, suggest that POA may induce modification in the epigenetics of aging oocytes, leading to poor fertilization rates and embryo development.
Even more profound effect of POA has been found on genomic constitution of mammalian oocytes. Due to the scarcity of human oocytes, animal models have been mostly used to accumulate the evidence on the importance of POA on decreased oocyte quality and compromised viability of in vitro produced embryos generated from POA-oocytes. As POA studies focus on the phenomenon occurring with the ovulated oocytes, the POA concerns mostly MII oocytes that have successfully completed the first meiotic division separating the paired homologous chromosomes into MII oocyte and PBI, thus halving the chromosomal count from diploidy to haploidy. In MII oocytes, the chromosomes are made of the two sister chromatids that are aligned on the MII metaphase plate. The sister chromatids that are connected by adhesion factor, referred as cohesin, are oriented toward opposite spindle poles in a bipolar fashion by kinetochore protein structure mediating the connection between the centromeric DNA and meiotic spindle microtubules. Spindle assembly checkpoint (SAC), which operates during MII divisions, is one of the cell cycle checkpoint mechanisms that ensures that chromosomes are correctly attached and aligned to the spindle and are subsequently evenly segregated between the daughter cells. Protein MAD2 must be targeted to sister chromatids kinetochores, in order to accelerate the attachment of chromosomes to the spindle microtubules that ensures the proper meiotic segregation of chromosomes. It has been shown that in murine POA-oocytes, MAD2 localization to the sister kinetochores is inhibited, thus also the chromosome spindle attachment may remain incomplete [10]. In addition, POA prevents cohesin from being maintained or degraded at the appropriate time, thus destabilizing the SAC signaling and causing sister chromatid segregation errors, consequently leading to the higher incidence of aneuploidy in early embryos.
The mechanisms of POA in human oocytes are significantly more difficult to study, because of ethical restrictions, as human mature oocytes cannot be used for research, at least not in sufficient quantities. However, the IVF procedure, as the most commonly practiced ART procedure, provides a good opportunity to better understand the mechanisms of POA. IVF clinics today are large medical facilities where procedures can be delayed due to the excessive workload of clinical and laboratory staff. In addition, there may be a situation where a semen sample arrives at the laboratory significantly later than expected. All of this can cause the delay between the oocyte retrieval and the planned timing of insemination or ICSI. The timing of performing the injection of oocytes after denudation has been shown to be a critical factor determining the pregnancy success of ICSI. Indeed, recently, the analysis of more than 3600 ICSI treatments revealed that a long time from denudation to ICSI was associated with significantly decreased clinical pregnancy rate when compared to the cycles with short interval of up to 4 h [111]. Although this study did not reveal any possible POA mechanisms, the results suggest that POA in oocytes, especially in artificial environment, begins fairly rapidly after oocyte retrieval and must be considered more carefully in clinical practice. In addition, the POA process may be accelerated by the fact that oocyte culture conditions in the IVF/ICSI procedure do not yet meet ideal physiological conditions and may therefore be one of the reasons for the acceleration of POA.
A more direct evidence of POA’s effect on the deteriorated oocyte quality has been provided by IVF procedures with unexpected fertilization failure. In these unfortunate cases, IVF is done on retrieved mature oocytes but without fertilization, while rescue ICSI is done after 24 h using in vitro aged oocytes. The pregnancy results following “rescue ICSI”-derived embryos have remained relatively modest, which likely indicates the deteriorating effect of in vitro POA on mature human oocytes. There is only a single report providing the information on chromosomal aberrations in these embryos disclosing the high rate of diverse chromosomal aberrations [112]. As a conclusion, the use of “rescue ICSI” embryos was discouraged due to the observed increase in chromosomal abnormalities in these embryos, likely manifesting the negative effect of POA on human embryos.
Environmental chemicals and oocyte quality
As discussed above, multiple chemicals that naturally occur in the environment, such as caffeine, resveratrol, and quercetin, have significant positive effects on oocyte quality in vitro and in vivo. Unfortunately, the environment also contains thousands of man-made chemicals [113], and some of them can disrupt the endocrine system being called endocrine-disrupting chemicals (EDCs) [114, 115]. Bisphenols and phthalates are examples of EDCs that are used in plastics and cosmetics, and perfluoroalkyl substances are surface-active chemicals used in food packaging, firefighting foam and non-sticky textiles, and kitchen utensils. Some man-made chemicals, such as chlorinated pesticides (e.g., DDT) and polychlorinated biphenyls (PCBs), have been restricted internationally due to their toxic properties, but they still widely contaminate food stock due to their extreme persistent. Ubiquitous exposure to pesticides, PCBs, phenols, fluoroalkyl compounds, flame retardants, and perchlorate can be found in Americans including pregnant mothers [116, 117]. Exposure starts in the womb, as EDCs can be found in umbilical cord blood and fetal tissues [110, 118, 119]. EDCs can also be detected in human ovarian follicular fluid; PCBs, pesticides, and PFAS are present virtually in all ART patients studied, and the levels in serum and follicular fluid are roughly the same [120]. High levels of PCBs in serum of women correlate with lower ovarian reserve, poorer embryo quality, and longer time-to-pregnancy, suggesting that chemical exposures can accelerate reproductive aging [121, 122]. Collectively, these results imply that chemical contaminants may affect oocyte quality, especially by impairing mitochondrial activity [123,124,125], thereby lessening the chances for fertilization. There are no studies using in vitro exposure of human oocytes to EDCs to investigate impact on maturation and POA, but animal studies strongly support the sensitivity of mammalian oocytes toward low-level EDC exposure. For example, exposure of bovine cumulus-oocyte-complexes during IVM to PFOS (a perfluoroalkyl substance used in firefighting foam) at levels found in human ovarian follicular fluid leads to a delayed cleavage development, altered lipid distribution, and epigenetic and gene expression changes that persist to blastocyst stage [126]. Similarly, exposure of mice to bisphenol A via damaged polycarbonate cages or oral exposure leads to congression failure in oocytes [127]. A realistic scenario is that the developmental competence of oocytes exposed for years to these chemicals can be dramatically impaired per se, and that it could be further worsened by the degenerative processes induced by POA, either in vivo or in vitro. Significant research remains to be accomplished, including studies of EDCs that may leak from plastics used during IVF treatments, to fully understand the consequences of man-made chemicals on POA.
Conclusions
Literature data clearly evidence that POA causes a number of morphological, molecular, genomic, and epigenomic aberrations in oocytes and related embryos, the mechanisms of which are beginning to be gradually acknowledged. However, it can be assumed that POA plays a major role in determining the impaired environment, by increasing ROS levels and oxidative stress, capable of affecting developmental potential of oocytes and embryos, pivotal in IVF/ICSI cycles. As genetic and epigenetic alterations may be passed on to the next generation and cause health issues and diseases, the mechanisms of in vitro POA should be investigated in much more detail in the future. The present review strongly suggests that avoiding POA and the use of “antiaging” molecules during in vitro culture may be key-elements to improve the overall performance of the IVF procedure.
References
Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod. 2013;88:67.
Zhu J, Zhang J, Li H, Wang TY, Zhang CX, Luo MJ, et al. Cumulus cells accelerate oocyte aging by releasing soluble Fas Ligand in mice. Sci Rep. 2015;5:8683.
Lord T, John Aitken R. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction. 2013;146(6):R217–27.
Zuccotti M, Merico V, Cecconi S, Redi CA, Garagna S. What does it take to make a developmentally competent mammalian egg? Hum Reprod Update. 2011;17:525–40.
Canipari R, Cellini V, Cecconi S. The ovary feels fine when paracrine and autocrine networks cooperate with gonadotropins in the regulation of folliculogenesis. Curr Pharm Des. 2012;18:245–55.
Sirait B, Wiweko B, Jusuf AA, Iftitah D, Muharam R. Oocyte competence biomarkers associated with oocyte maturation: a review. Front Cell Dev Biol. 2021;9:710292.
Takai Y, Matikainen T, Jurisicova A, Kim MR, Trbovich AM, Fujita E, et al. Caspase-12 compensates for lack of caspase-2 and caspase-3 in female germ cells. Apoptosis. 2007;12:791–800.
Premkumar KV, Chaube SK. An insufficient increase of cytosolic free calcium level results postovulatory aging-induced abortive spontaneous egg activation in rat. J Assist Reprod Genet. 2013;30:117–23.
Prasad S, Tiwari M, Koch B, Chaube SK. Morphological, cellular and molecular changes during postovulatory egg aging in mammals. J Biomed Sci. 2015;22:1–5.
Shimoi G, Tomita M, Kataoka M, Kameyama Y. Destabilization of spindle assembly checkpoint causes aneuploidy during meiosis II in murine post-ovulatory aged oocytes. J Reprod Dev. 2019;65:57–66.
Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update. 2009;15:573–85.
Tripathi A, Chaube SK. Reduction of phosphorylated Thr-161 Cdk1 level participates in roscovitine-induced Fas ligand-mediated apoptosis in rat eggs cultured in vitro. In Vitro Cell Dev Biol. 2015;51:174–82.
Arroyo A, Kim B, Yeh J. Luteinizing hormone action in human oocyte maturation and quality: signaling pathways, regulation, and clinical impact. Reprod Sci. 2020;27:1223–52.
Zhang M, Su Y-Q, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:362–6.
Liu W, Xin Q, Wang X, Wang S, Wang H, Zhang W, et al. Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals. Cell Death Dis. 2017;8:e2662.
Wang X, Wang H, Liu W, Zhang Z, Zhang Y, Zhang W, et al. High level of C-type natriuretic peptide induced by hyperandrogen-mediated anovulation in polycystic ovary syndrome mice. Clin Sci. 2018;132:759–76.
Pan B, Li J. The art of oocyte meiotic arrest regulation. Reprod Biol Endocrinol. 2019;17:8.
Hao X, Wang Y, Kong N, Zhang Y, Zhao Y, Xia G, et al. Epidermal growth factor-mobilized intracellular calcium of cumulus cells decreases natriuretic peptide receptor 2 affinity for natriuretic peptide type C and induces oocyte meiotic resumption in the mouse. Biol Reprod. 2016;95:45.
Richani D, Gilchrist RB. The epidermal growth factor network: role in oocyte growth, maturation and developmental competence. Hum Reprod Update. 2018;24:1–14.
Fan H-Y, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, et al. MAPK3/1 (ERK1/2) in Ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–41.
Sha QQ, Zhang J, Fan HY. Function and regulation of histone H3 lysine-4 methylation during oocyte meiosis and maternal-to-zygotic transition. Front Cell Dev Biol. 2020;8:597498.
Huang CJ, Wu D, Jiao XF, Khan FA, Xiong CL, Liu XM, et al. Maternal SENP7 programs meiosis architecture and embryo survival in mouse. Biochim Biophys Acta Mol Cell Res. 2017;1864:1195–206.
Jones KT. Anaphase-promoting complex control in female mouse meiosis. In: Kubiak JZ, editor. Cell Cycle in Development. Berlin, Heidelberg: Springer; 2011. p. 343–63.
Madgwick S, Jones KT. How eggs arrest at metaphase II: MPF stabilisation plus APC/C inhibition equals cytostatic factor. Cell Div. 2007;2:4.
Fujioka YA, Onuma A, Fujii W, Sugiura K, Naito K. Contributions of UBE2C and UBE2S to meiotic progression of porcine oocytes. J Reprod Dev. 2018;64:253–9.
Wang Y, Schatten H, Cui XS, Sun SC. Editorial: quality control of mammalian oocyte meiotic maturation: causes, molecular mechanisms and solutions. Front Cell Dev Biol. 2021;9:736331.
Zhang L, Wang Z, Lu T, Meng L, Luo Y, Fu X, et al. Mitochondrial Ca2+ overload leads to mitochondrial oxidative stress and delayed meiotic resumption in mouse oocytes. Front Cell Dev Biol. 2020;8:580876.
Hatırnaz Ş, Ata B, Hatırnaz ES, Dahan MH, Tannus S, Tan J, et al. Oocyte in vitro maturation: a sytematic review. Turk J Obstet Gynecol. 2018;15:112–25.
Coticchio G. IVM in need of clear definitions. Hum Reprod. 2016;31:1387–9.
Kuhtz J, Romero S, de Vos M, Smitz J, Haaf T, Anckaert E. Human in vitro oocyte maturation is not associated with increased imprinting error rates at LIT1, SNRPN, PEG3 and GTL2. Hum Reprod. 2014;29:1995–2005.
Pliushch G, Schneider E, Schneider T, el Hajj N, Rösner S, Strowitzki T, et al. In vitro maturation of oocytes is not associated with altered deoxyribonucleic acid methylation patterns in children from in vitro fertilization or intracytoplasmic sperm injection. Fertil Steril. 2015;103:720-727.e1.
Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod. 1998;13:394–7.
Díaz H, Esponda P. Ageing-induced changes in the cortical granules of mouse eggs. Zygote. 2004;12:95–103.
Szollosi D. Morphological changes in mouse eggs due to aging in the fallopian tube. Am J Anat. 1971;130:209–25.
Szollosi D. Mammalian eggs aging in the fallopian tubes. Aging Gametes Int Symp. Karger; 1973. p. 98–121.
Zheng J, Yin XQ, Ge W, He GF, Qian WP, Ma JY, et al. Post-ovulatory aging of mouse oocytes in vivo and in vitro: effects of caffeine on exocytosis and translocation of cortical granules. Anim Sci J. 2016;87:1340–6.
Xu Z, Abbott A, Kopf GS, Schultz RM, Ducibella T. Spontaneous activation of ovulated mouse eggs: time-dependent effects on M-phase exit, cortical granule exocytosis, maternal messenger ribonucleic acid recruitment, and inositol 1,4,5-trisphosphate sensitivity’. Biol Reprod. 1997;57:743–50.
Goud AP, Goud PT, Diamond MP, van Oostveldt P, Hughes MR. Microtubule turnover in ooplasm biopsy reflects ageing phenomena in the parent oocyte. Reprod BioMed Online. 2005;11:43–52.
Miao YL, Liu XY, Qiao TW, Miao DQ, Luo MJ, Tan JH. Cumulus cells accelerate aging of mouse oocytes. Biol Reprod. 2005;73:1025–31.
Liang QX, Lin YH, Zhang CH, Sun HM, Zhou L, Schatten H, et al. Resveratrol increases resistance of mouse oocytes to postovulatory aging in vivo. Aging. 2018;10:1586–96.
Wortzman GB, Evans JP. Membrane and cortical abnormalities in post-ovulatory aged eggs: analysis of fertilizability and establishment of the membrane block to polyspermy. Mol Hum Reprod. 2005;11:1–9.
Szpila M, Walewska A, Sabat-Pośpiech D, Strączyńska P, Ishikawa T, Milewski R, et al. Postovulatory ageing modifies sperm-induced Ca2+ oscillations in mouse oocytes through a conditions-dependent, multi-pathway mechanism. Sci Rep. 2019;9:11859.
Zenzes MT, Casper RF. Cytogenetics of human oocytes, zygotes, and embryos after in vitro fertilization. Hum Genet. 1992;88:367–75.
Mackenzie ACL, Kyle DD, McGinnis LA, Lee HJ, Aldana N, Robinson DN, et al. Cortical mechanics and myosin-II abnormalities associated with postovulatory aging: Implications for functional defects in aged eggs. Mol Hum Reprod. 2016;22:397–409.
Dalo DT, Mccaffery JM, Evans JP. Ultrastructural analysis of egg membrane abnormalities in post-ovulatory aged eggs. Int J Dev Biol. 2008;52:535–44.
Sun GY, Gong S, Kong QQ, Li ZB, Wang J, Xu MT, et al. Role of AMP-activated protein kinase during postovulatory aging of mouse oocytes. Biol Reprod. 2020;103:534–47.
Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–89.
Ozawa M, Matsuzuka T, Hirabayashi M, Kanai Y. Redox status of the oviduct and Cdc2 activity in 2-cell stage embryos in heat-stressed mice. Biol Reprod. 2004;71:291–6.
Igarashi H, Takahashi T, Takahashi E, Tezuka N, Nakahara K, Takahashi K, et al. Aged mouse oocytes fail to readjust intracellular adenosine triphosphates at fertilization. Biol Reprod. 2005;72:1256–61.
Igarashi H, Takahashi T, Abe H, Nakano H, Nakajima O, Nagase S. Poor embryo development in post-ovulatory in vivo-aged mouse oocytes is associated with mitochondrial dysfunction, but mitochondrial transfer from somatic cells is not sufficient for rejuvenation. Hum Reprod. 2016;31:2331–8.
Rodríguez-Varela C, Herraiz S, Labarta E. Mitochondrial enrichment in infertile patients: a review of different mitochondrial replacement therapies. Ther Adv Reprod Health. 2021;15:263349412110235.
Di Emidio G, Falone S, Vitti M, D’Alessandro AM, Vento M, Di Pietro C, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29:2006–17.
Wang F, Nguyen M, Qin FXF, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–14.
Zhang L, Han L, Ma R, Hou X, Yu Y, Sun S, et al. Sirt3 prevents maternal obesity-associated oxidative stress and meiotic defects in mouse oocytes. Cell Cycle. 2015;14:2959–68.
Zhang T, Zhou Y, Li L, Wang H, Ma X, Qian W, et al. SIRT1, 2, 3 protect mouse oocytes from postovulatory aging. Aging. 2016;8:685–94.
Takehara I, Igarashi H, Kawagoe J, Matsuo K, Takahashi K, Nishi M, et al. Impact of endoplasmic reticulum stress on oocyte aging mechanisms. Mol Hum Reprod. 2020;26:567–75.
Szpila M, Walewska A, Sabat-Pośpiech D, Strączyńska P, Ishikawa T, Milewski R, et al. Postovulatory ageing modifies sperm-induced Ca2+ oscillations in mouse oocytes through a conditions-dependent, multi-pathway mechanism. Sci Rep. 2019;9:1–18.
Yuan R-Y, Wang F, Li S, Ma J-Y, Guo L, Li X-L, et al. Maturation conditions, post-ovulatory age, medium pH, and ER stress affect [Ca 2+ ]i oscillation patterns in mouse oocytes. J Assist Reprod Genet. 2021;38:1373–85.
Takahashi T, Saito H, Hiroi M, Doi K, Takahashi E. Effects of aging on inositol 1,4,5-triphosphate-induced Ca 2 release in unfertilized mouse oocytes. Mol Reprod Dev. 2000;55:299–306.
Zhang N, Wakai T, Fissore RA. Caffeine alleviates the deterioration of Ca 2+ release mechanisms and fragmentation of in vitro-aged mouse eggs. Mol Reprod Dev. 2011;78:684–701.
Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003;66:143–52.
Goud PT, Goud AP, Laverge H, de Sutter P, Dhont M. Effect of post-ovulatory age and calcium in the injection medium on the male pronucleus formation and metaphase entry following injection of human spermatozoa into golden hamster oocytes. Mol Hum Reprod. 1999;5:227–33.
Abbott AL, Xu Z, Kopf GS, Ducibella T, Schultz RM. In vitro culture retards spontaneous activation of cell cycle progression and cortical granule exocytosis that normally occur in vivo unfertilized mouse eggs. Biol Reprod. 1998;59:1515–21.
Jin YX, Cui XS, Yu XF, Han YJ, Kong IK, Kim NH. Alterations of spindle and microfilament assembly in aged cat oocytes. Reprod Domest Anim. 2010;45:865–71.
Cecconi S, Rossi G, Deldar H, Cellini V, Patacchiola F, Carta G, et al. Post-ovulatory ageing of mouse oocytes affects the distribution of specific spindle-associated proteins and Akt expression levels. Reprod Fertil Dev. 2014;26:562–9.
Cecconi S, Mauro A, Cellini V, Patacchiola F. The role of Akt signalling in the mammalian ovary. Int J Dev Biol. 2012;56:809–17.
Steuerwald NM, Steuerwald MD, Mailhes JB. Post-ovulatory aging of mouse oocytes leads to decreased MAD2 transcripts and increased frequencies of premature centromere separation and anaphase. Mol Hum Reprod. 2005;11:623–30.
Jeon HJ, Cui XS, Guo J, Lee JM, Kim JS, Oh JS. TCTP regulates spindle assembly during postovulatory aging and prevents deterioration in mouse oocyte quality. Biochim Biophys Acta Mol Cell Res. 2017;1864:1328–34.
Takahashi T, Igarashi H, Amita M, Hara S, Matsuo K, Kurachi H. Molecular mechanism of poor embryo development in postovulatory aged oocytes: mini review. J Obstet Gynaecol Res. 2013;39:1431–9.
Wang TY, Zhang J, Zhu J, Lian HY, Yuan HJ, Gao M, et al. Expression profiles and function analysis of microRNAs in postovulatory aging mouse oocytes. Aging. 2017;9:1186–201.
Willimott S, Wagner SD. Post-transcriptional and post-translational regulation of Bcl2. Biochem Soc Trans. 2010;38(6):1571–5.
Yang Q, Dai S, Luo X, Zhu J, Li F, Liu J, et al. Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression. Reproduction. 2018;156:81–92.
Lin FH, Zhang WL, Li H, Tian XD, Zhang J, Li X, et al. Role of autophagy in modulating post-maturation aging of mouse oocytes. Cell Death Dis. 2018;9:308.
Ge ZJ, Schatten H, Zhang CL, Sun QY. Oocyte ageing and epigenetics. Reproduction. 2015;149:R103–14.
Wang T, Gao YY, Chen L, Nie ZW, Cheng W, Liu X, et al. Melatonin prevents postovulatory oocyte aging and promotes subsequent embryonic development in the pig. Aging. 2017;9:1552–64.
Abbasi B, Dong Y, Rui R. Resveratrol hinders postovulatory aging by modulating oxidative stress in porcine oocytes. Molecules. 2021;26:6346.
Kim N-H, Moon JU, Prather RS, Day BN. Cytoskeletal alteration in aged porcine oocytes and parthenogenesis. Mol Reprod Dev. 1996;43:513–8.
Bianchi S, Macchiarelli G, Micara G, Linari A, Boninsegna C, Aragona C, et al. Ultrastructural markers of quality are impaired in human metaphase II aged oocytes: a comparison between reproductive and in vitro aging. J Assist Reprod Genet. 2015;32:1343–58.
Wang Y, Li L, Fan LH, Jing Y, Li J, Ouyang YC, et al. N-acetyl-L-cysteine (NAC) delays post - ovulatory oocyte aging in mouse. Aging. 2020;11:2020–30.
Wang HY, Jo YJ, Oh JS, Kim NH. Quercetin delays postovulatory aging of mouse oocytes by regulating SIRT expression and MPF activity. Oncotarget. 2017;8:38631–41.
Jeon HJ, You SY, Kim DH, Jeon HB, Oh JS. Protective effects of ethanol extracts of Artemisia asiatica Nakai ex Pamp. on ageing-induced deterioration in mouse oocyte quality. Zygote. 2017;25:472–9.
Yoon JW, Lee SE, Park YG, Kim WJ, Park HJ, Park CO, et al. The antioxidant icariin protects porcine oocytes from age-related damage in vitro. Anim Biosci. 2021;34:546–57.
Xu W, Li L, Sun J, Zhu S, Yan Z, Gao L, et al. Putrescine delays postovulatory aging of mouse oocytes by upregulating PDK4 expression and improving mitochondrial activity. Aging. 2018;10:4093–106.
Lord T, Martin JH, Aitken RJ. Accumulation of electrophilic aldehydes during postovulatory aging of mouse oocytes causes reduced fertility, oxidative stress, and apoptosis. Biol Reprod. 2015;92:33.
Li Q, Cui LB. Combined inhibitory effects of low temperature and N-acetyl-L-cysteine on the postovulatory aging of mouse oocytes. Zygote. 2016;24:195–205.
Petri T, Dankert D, Demond H, Wennemuth G, Horsthemke B, Grümmer R. In vitro postovulatory oocyte aging affects H3K9 trimethylation in two-cell embryos after IVF. Ann Anat. 2020;227:151424.
Imamura T, Kerjean A, Heams T, Kupiec JJ, Thenevin C, Pàldi A. Dynamic CpG and non-CpG methylation of the Peg1/Mest gene in the mouse oocyte and preimplantation embryo. J Biol Chem. 2005;280:20171–5.
Liang XW, Zhu JQ, Miao YL, Liu JH, Wei L, Lu SS, et al. Loss of methylation imprint of Snrpn in postovulatory aging mouse oocyte. Biochem Biophys Res Commun. 2008;371:16–21.
MCGinnis LK, Pelech S, Kinsey WH. Post-ovulatory aging of oocytes disrupts kinase signaling pathways and lysosome biogenesis. Mol Reprod Dev. 2014;81:928–45.
Miao Y, Zhou C, Cui Z, Zhang M, ShiYang X, Lu Y, et al. Postovulatory aging causes the deterioration of porcine oocytes via induction of oxidative stress. FASEB J. 2018;32:1328–37.
Dai X, Lu Y, Zhang M, Miao Y, Zhou C, Cui Z, et al. Melatonin improves the fertilization ability of post-ovulatory aged mouse oocytes by stabilizing ovastacin and Juno to promote sperm binding and fusion. Hum Reprod. 2017;32:598–606.
Zhou J, Xue Z, He HN, Liu X, Yin SY, Wu DY, et al. Resveratrol delays postovulatory aging of mouse oocytes through activating mitophagy. Aging. 2019;11:11504–19.
Sun YL, Tang Sb, Shen W, Yin S, Sun QY. Roles of resveratrol in improving the quality of postovulatory aging oocytes in vitro. Cells. 2019;8(10):1132.
Zhang M, ShiYang X, Zhang Y, Miao Y, Chen Y, Cui Z, et al. Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis. Free Radic Biol Med. 2019;143:84–94.
Jia BY, Xiang DC, Shao QY, Zhang B, Liu SN, Hong QH, et al. Inhibitory effects of astaxanthin on postovulatory porcine oocyte aging in vitro. Sci Rep. 2020;10:20217.
Niu YJ, Zhou W, Nie ZW, Zhou D, Xu YN, Ock SA, et al. Ubiquinol-10 delays postovulatory oocyte aging by improving mitochondrial renewal in pigs. Aging. 2020;12:1256–71.
Ogawa T, Fukasawa H, Hirata S. Improvement of early developmental competence of postovulatory-aged oocytes using metaphase II spindle injection in mice. Reprod Med Biol. 2020;19:357–64.
Kim JY, Zhou D, Cui XS. Bezafibrate prevents aging in in vitro-matured porcine oocytes. J Anim Sci Technol. 2021;63:766–77.
Tang DW, Fang Y, Liu ZX, Wu Y, Wang XL, Zhao S, et al. The disturbances of endoplasmic reticulum calcium homeostasis caused by increased intracellular reactive oxygen species contributes to fragmentation in aged porcine oocytes. Biol Reprod. 2013;89:124.
Tatone C, di Emidio G, Barbaro R, Vento M, Ciriminna R, Artini PG. Effects of reproductive aging and postovulatory aging on the maintenance of biological competence after oocyte vitrification: Insights from the mouse model. Theriogenology. 2011;76:864–73.
Luo D, Zhang JB, Li SP, Liu W, Yao XR, Guo H, et al. Imperatorin ameliorates the aging-associated porcine oocyte meiotic spindle defects by reducing oxidative stress and protecting mitochondrial function. Front Cell Dev Biol. 2020;8:592433.
Prasad S, Koch B, Chaube SK. RO-3306 prevents postovulatory aging-mediated spontaneous exit from M-II arrest in rat eggs cultured in vitro. Biomed Pharmacother. 2016;78:216–25.
Tatone C, Carbone MC, Gallo R, Delle Monache S, di Cola M, Alesse E, et al. Age-associated changes in mouse oocytes during postovulatory in vitro culture: possible role for meiotic kinases and survival factor BCL2. Biol Reprod. 2006;74:395–402.
Ma W, Zhang D, Hou Y, Li YH, Sun QY, Sun XF, et al. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol Reprod. 2005;72:373–83.
Trapphoff T, Heiligentag M, Dankert D, Demond H, Deutsch D, Fröhlich T, et al. Postovulatory aging affects dynamics of mRNA, expression and localization of maternal effect proteins, spindle integrity and pericentromeric proteins in mouse oocytes. Hum Reprod. 2016;31:133–49.
Huang JC, Yan LY, Lei ZL, Miao YL, Shi LH, Yang JW, et al. Changes in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod. 2007;77:666–70.
Liu N, Wu YG, Lan GC, Sui HS, Ge L, Wang JZ, et al. Pyruvate prevents aging of mouse oocytes. Reproduction. 2009;138:223–34.
Lee AR, Thanh Ha L, Kishigami S, Hosoi Y. Abnormal lysine acetylation with postovulatory oocyte aging. Reprod Med Biol. 2014;13:81–6.
Dankert D, Demond H, Trapphoff T, Heiligentag M, Rademacher K, Eichenlaub-Ritter U, et al. Pre- and postovulatory aging of murine oocytes affect the transcript level and poly(A) tail length of maternal effect genes. PLoS ONE. 2014;9(10):e108907.
Panagopoulos Abrahamsson D, Wang A, Jiang T, Wang M, Siddharth A, Morello-Frosch R, et al. A comprehensive non-targeted analysis study of the prenatal exposome. Environ Sci Technol. 2021;55:10542–57.
Zhang Y, Ma Y, Fang Z, Hu S, Li Z, Zhu L, et al. Performing ICSI within 4 hours after denudation optimizes clinical outcomes in ICSI cycles. Reprod Biol Endocrinol. 2020;18:27.
Pehlivan T, Rubio C, Ruiz A, Navarro J, Remohí J, Pellicer A, et al. Embryonic chromosomal abnormalities obtained after rescue intracytoplasmic sperm injection of 1-day-old unfertilized oocytes. J Assist Reprod Genet. 2004;21:55–7.
Wang Z, Walker GW, Muir DCG, Nagatani-Yoshida K. Toward a global understanding of chemical pollution: a first comprehensive analysis of national and regional chemical inventories. Environ Sci Technol. 2020;54:2575–84.
World Health Organization, United Nations Environment Programme, Inter-Organization Programme for the Sound Management of Chemicals, Bergman A, Heindel JJ, Jobling S, et al. State of the science of endocrine disrupting chemicals 2012: summary for decision-makers. World Health Organization (WHO); 2013.
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:E1-150.
Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assists environmental medicine physicians. Altern Med Rev. 2010;15:101–8.
Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the united states: NHANES 2003–2004. Environ Health Perspect. 2011;119:878–85.
Mamsen LS, Björvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, et al. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int. 2019;124:482–92.
Björvang RD, Vinnars MT, Papadogiannakis N, Gidlöf S, Mamsen LS, Mucs D, et al. Mixtures of persistent organic pollutants are found in vital organs of late gestation human fetuses. Chemosphere. 2021;283:131125.
Björvang RD, Damdimopoulou P. Persistent environmental endocrine-disrupting chemicals in ovarian follicular fluid and in vitro fertilization treatment outcome in women. Ups J Med Sci. 2020;125:85–94.
Björvang RD, Hassan J, Stefopoulou M, Gemzell-Danielsson K, Pedrelli M, Kiviranta H, et al. Persistent organic pollutants and the size of ovarian reserve in reproductive-aged women. Environ Int. 2021;155:106589.
Björvang RD, Gennings C, Lin PI, Hussein G, Kiviranta H, Rantakokko P, et al. Persistent organic pollutants, pre-pregnancy use of combined oral contraceptives, age, and time-to-pregnancy in the SELMA cohort. Environ Health. 2020;19:67.
Rossi G, Palmerini MG, Macchiarelli G, Buccione R, Cecconi S. Mancozeb adversely affects meiotic spindle organization and fertilization in mouse oocytes. Reprod Toxicol. 2006;22:51–5.
Rossi G, Buccione R, Baldassarre M, MacChiarelli G, Palmerini MG, Cecconi S. Mancozeb exposure in vivo impairs mouse oocyte fertilizability. Reprod Toxicol. 2006;21:216–9.
Iorio R, Castellucci A, Rossi G, Cinque B, Cifone MG, Macchiarelli G, et al. Mancozeb affects mitochondrial activity, redox status and ATP production in mouse granulosa cells. Toxicol In Vitro. 2015;30:438–45.
Hallberg I, Persson S, Olovsson M, Sirard M-A, Damdimopoulou P, Rüegg J, et al. Perfluorooctane sulfonate (PFOS) exposure of bovine oocytes affects early embryonic development at human-relevant levels in an in vitro model. Toxicology. 2021;464:153028.
Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, et al. Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–53.
Funding
Open access funding provided by Università degli Studi dell’Aquila within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Consortia
Contributions
SC, SA, and VDN contributed to the conception and design of the review article. All the authors drafted the article, critically revised the work, and approved the final version of the review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di Nisio, V., Antonouli, S., Damdimopoulou, P. et al. In vivo and in vitro postovulatory aging: when time works against oocyte quality?. J Assist Reprod Genet 39, 905–918 (2022). https://doi.org/10.1007/s10815-022-02418-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02418-y