Abstract
Background
Polycystic ovary syndrome (PCOS) is a heterogeneous endocrinopathy and a leading cause of anovulatory infertility. Angiogenesis is vital for ovarian folliculogenesis. The expression of angiogenesis-associated genes/proteins is altered in the ovary of PCOS women. However, information on microRNAs (miRNAs) regulating their expression is limited. This study aims to identify dysregulated angiogenesis-related genes in the ovary of women with PCOS, to identify miRNAs regulating them, and to construct a miRNA-mRNA network associated with angiogenesis.
Methods
A comprehensive literature search and reanalysis of seven ovarian GEO microarray datasets were performed to identify differentially expressed angiogenesis-related genes in PCOS. These target genes were used to predict their regulating miRNAs by querying miRNA databases and their expression in the ovary was verified. Panther and STRING database were used for functional enrichment. Gene expression of shortlisted miRNAs was studied in granulosa cells using digital droplet PCR.
Results
The miRNAs expressed in the ovary and potentially targeting dysregulated angiogenesis-related genes in PCOS were identified and those enriched in angiogenesis-related pathways, like VEGF, FGF, PI3K/Akt, Notch signaling, and ECM interaction were shortlisted. Analysis showed PI3K/Akt signaling was the most enriched pathway. MiR-218-5p, miR-214-3p, miR-20a-5p, and miR-140-3p associated with the PI3K/Akt pathway were found to be up-regulated in granulosa cells of women with PCOS.
Conclusions
By in silico analysis, we identified crucial dysregulated angiogenesis-related genes, the miRNA-mRNA interactions, and signaling pathways involved in impaired follicular angiogenesis in PCOS. This work provides a novel insight into the mechanism of aberrant ovarian angiogenesis contributing to PCOS pathophysiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is the most prevalent multifactorial endocrine and metabolic disorder in reproductive-age women. The characteristic features of PCOS are menstrual irregularities, anovulation, increased gonadotropin-releasing hormone (GnRH), pulsatility, and luteinizing hormone (LH) hypersecretion, hyperandrogenemia, obesity, and polycystic ovaries. PCOS is one of the leading causes of anovulatory infertility. It is also associated with an increased risk of insulin resistance and compensatory hyperinsulinemia, type 2 diabetes mellitus, and cardiovascular diseases [1]. Follicle development is a sequential process that begins with the recruitment of primary follicles from the primordial follicle pool, growth and development of follicles, selection of dominant follicle, and ovulation and formation of corpus luteum (CL). Several processes like hormone production, angiogenesis, extracellular matrix remodeling, COC matrix expansion, and luteolysis act in a synchronized fashion during folliculogenesis. Alteration of any of these processes may lead to ovulatory dysfunction and few of these processes are compromised in women with PCOS [2,3,4].
Angiogenesis is a process by which new blood vessels are formed in the ovary which ensures proper supply of nutrients and hormones to the growing follicle and the formation and maintenance of the CL. The vascularization of the pre-existing endothelial cells in the theca cell layer is induced by several growth factors, mainly secreted by granulosa cells (GCs) [5]. These factors have been reported to be crucial to promote vessel permeability, thus favoring the antrum formation and the events inducing follicle rupture. After ovulation, the basement membrane breaks down, the theca cells along with the blood vessels enter the follicle and intermingle with GCs to form highly vascularized CL. In women with PCOS, CL insufficiency has been reported which may be the cause of decreased progesterone levels and frequent miscarriages experienced by these women [3, 6, 7]. A recent study demonstrated altered expression of angiogenic genes in GCs and follicular fluid, reduced angiogenic capacity of follicular fluid and endothelial cell–like property of the GCs in women with PCOS indicating dysregulated angiogenesis which may explain the compromised CL function in these women [3].
Several reports have emphasized the altered expression of various angiogenic proteins and genes like vascular endothelial growth factor (VEGF), angiopoietins (ANGPT), fibroblast growth factor (FGF), platelet-derived growth factors (PDGF), hypoxia inducing factor 1A (HIF1A), and tumor necrosis factor in the ovary of women with PCOS [3, 8]. However, regulation of these differentially expressed genes related to angiogenesis has not been explored. Despite much information available on PCOS, follicular angiogenesis in the ovary is not studied in detail yet.
Gene expression is regulated by micro-RNAs (miRNAs) which are small (≈ 22nt), evolutionarily conserved, and single-stranded non-coding RNAs that mostly mediate gene silencing at the post-transcriptional level by mRNA degradation and/or translational repression by binding to 3’ UTR of their target mRNA. Also, the binding of miRNAs to 5’ UTR and coding or promoter region suppresses the gene expression [9]. The genes in multiple biological processes like cell division, differentiation, apoptosis, and angiogenesis are under the control of miRNAs [10,11,12]. Studies have also reported the involvement of miRNA in the regulation of ovarian follicle growth and development, steroidogenesis, ovulation, and CL formation [13,14,15]. Thus, the involvement of miRNAs in the angiogenesis process and ovarian follicle development made them ideal candidates for investigating their role in follicular angiogenesis in PCOS. The information on unique molecular profiles of miRNAs involved in the regulation of angiogenesis in PCOS can potentiate further development of therapeutic agents and treatment of PCOS.
This study aimed to identify the miRNAs which target the genes related to angiogenesis which are differentially expressed in the ovary of women with PCOS. Towards this, we extensively searched literature and also reanalyzed the microarray expression datasets to enlist differentially expressed angiogenic genes in ovarian tissue of women with PCOS. Subsequently, these target genes were used as a seed dataset to obtain their interacting experimentally validated predicted miRNAs. Furthermore, the miRNAs reported to be expressed in the ovary of women with PCOS were enlisted by literature survey and compared with predicted miRNAs, to obtain overlapping miRNAs. Thus, this study generated an integrated miRNA-mRNA network which may have a role in altered angiogenesis in PCOS. Finally, we identified the list of potential miRNAs regulating angiogenesis in PCOS and inferred signaling pathways and potential mechanisms involved in PCOS pathophysiology.
Materials and methods
The methodology employed in this study is illustrated in Fig. 1. The study is approved by the Institutional Ethics Committee of Indian Council of Medical Research-National Institute for Research in Reproductive Health, Mumbai, India (ICMR-NIRRH) (Ethical approval no: 283/2015). The study was conducted as per the relevant guidelines and ethical norms. All study participants provided signed informed consent.
Literature search for identification of genes/proteins and miRNAs in women with PCOS
Separate searches were carried out to screen the genes/proteins associated with angiogenesis and miRNAs in PCOS independently. The PubMed [16] database (cited 9 July 2021) was searched using the query (Angiogen* AND (PCOS OR “Polycystic ovary syndrome”)). Human studies comparing the expression of genes in the ovary, ovarian cells or tissues, and follicular fluid in women with PCOS and controls were shortlisted. The studies carried out in animals, cell lines, review articles, articles in a non-English language, and articles without experimental validation were excluded. The full text of the shortlisted PubMed references was further mined for experimentally validated differential angiogenic genes/proteins.
Similarly, miRNAs reported in PCOS were retrieved from the PubMed database (cited 5 April 2021) using a combined search query ((“microRNA” OR "miR" OR “miRNA”) AND (PCOS OR “Polycystic ovary syndrome”)). Inclusion and exclusion criteria for article selection were the same as mentioned above. This miRNA dataset will be referred to henceforth as the “PCOS miRNAs dataset”
Microarray data analysis
The GEO (http://ncbi.nlm.nih.gov/geo/) [17] database was searched for expression profiling datasets comparing the differences in gene expression in ovary between women with PCOS and controls. The microarray datasets were analyzed by R-4.0.2 using the Bioconductor and Limma packages. Raw CEL files were downloaded for expression datasets done on the Affymetrix platform and were independently normalized using the robust multiarray average method [18]. In the case of Agilent arrays, the series matrix files were downloaded and quantile normalized. Probes matching with the same gene were collapsed and unmapped probes were removed. The genes were tested for differential expression using the Limma package [19]. Multiple testing correction was done by the Benjamini and Hochberg method. A log fold change of 1.5 and a P-value < 0.05 were set as cut-off criteria to identify differentially expressed genes (DEGs). The DEGs obtained from each dataset were analyzed by the PANTHER classification system (http://www.pantherdb.org/; accessed on: July 2021) to identify genes with a potential role in angiogenesis. The genes mapped to the angiogenesis, FGF-signaling, and VEGF signaling pathways were taken further for identification of their interacting miRNAs.
Retrieving miRNAs regulating the shortlisted target genes
The lists of differential angiogenesis-related genes in PCOS ovary identified from literature search and microarray data analysis were pooled together. The final dataset after removal of duplicate gene entries is referred to as “angiogenic dataset.” This list was further used to identify miRNA-mRNA interactions from miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/, release 7.0, Accessed on 13th July 2021) [20] and DIANA-TarBase V8 databases (https://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=tarbasev8%2Findex, accessed on: 13 July 2021) [21]. The interactions supported by strong experimental evidence and validated by low throughput studies were retained for further analysis. The lists of miRNA-mRNA interactions from both the databases were pooled and duplicates were removed and formed the “miRNA-mRNA dataset.” The unique miRNAs from this list were overlapped with the “PCOS miRNA dataset” to obtain “common miRNAs.”
Pathway analysis of miRNAs in PCOS
These common miRNAs identified in the above step were analyzed using the functional Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment feature in miEAA 2.0 (https://ccb-compute2.cs.uni-saarland.de/mieaa2/; accessed on 13 July 2021). Species were set to Homo sapiens, P-value adjustment method to Benjamini–Hochberg correction, and P-value cut-off to < 0.05. Heatmap showing distribution of miRNAs in the angiogenic pathway was constructed using a web-based tool, Morpheus (https://software.broadinstitute.org/morpheus/; accessed on July 2021).
Identification of functionally relevant interacting clusters in angiogenic gene dataset
The protein–protein interaction network for the “angiogenic dataset” was retrieved from the STRING database [22]. The interaction network was exported to Cytoscape software [23] for further analysis. The ClueGO plug-in in Cytoscape was used to identify interacting enriched pathway clusters associated with PCOS. The gene clusters associated with pathways involved in angiogenesis were analyzed further.
Selection of miRNAs for validation
The objective of this study was to identify novel candidate miRNAs with a predicted role in angiogenesis in PCOS. Hence, experimentally validated miRNAs reported in the ovary of women with PCOS were filtered out to obtain a final list of candidate miRNAs with a predicted role in angiogenesis in PCOS. Based on strength of existing evidence in literature and studies in the lab, four miRNAs were selected from the enriched pathways for experimental validation.
Study subjects and sample collection
Validation of selected miRNAs was carried out in GCs collected from women with PCOS and controls undergoing in vitro fertilization (IVF). The study was conducted at our institute of ICMR-NIRRH, Mumbai, India after obtaining ethical permission. The participants were recruited from Mumbai Fertility Clinic & IVF Center, Mumbai, India. All participants underwent controlled ovarian stimulation using GnRH antagonist protocol. From day 2 of the menses, recombinant follicle-stimulating hormone (rFSH, Gonal F, Merck, Germany) treatment was initiated with a dose of 150–300 units for the controls and 150–225 units for the PCOS group. Once the diameter of at least one follicle reaches 14 mm, GnRH antagonist (Orgalutran 0.25 mg, Organon, MSD, India) was injected daily. Both rFSH and antagonist were given daily till there were 3 or more follicles with a diameter of 18 mm. Oocyte maturation/follicular aspiration was timed by an intramuscular injection of recombinant human chorionic gonadotropin (rhCG) 5000 IU and Decapeptyl 0.1 mg and oocyte retrieval was performed 34–36 under ultrasound guidance. We recruited women with PCOS (n = 15) as per the Rotterdam consensus criteria. [24]. The healthy, age-BMI-matched regularly menstruating women who underwent IVF as oocyte donors were recruited as controls (n = 15). On the day of ovum pick-up (d-OPU), before administration of anesthesia, blood was collected from all recruited women. During oocyte retrieval, macroscopically clear follicular fluid was collected and processed as described earlier [25]. Baseline hormonal estimates for LH, FSH, prolactin, and TSH were obtained from clinical records, while serum and follicular fluid were used to assay estradiol (E2), progesterone (P4), total testosterone (TT), and sex hormone–binding globulin (SHBG) by electro-chemiluminescence technology using Roche e411 automated analyzer (Roche, Basel, Switzerland). Baseline levels for androgen excess indices were calculated using values of TT and SHBG [26].
Isolation and miRNA extraction from granulosa cells
The GCs were isolated from the follicular fluid as described previously [3]. Briefly, GCs were purified by centrifuging through a Ficoll gradient (HiMedia, India) and used for miRNA expression analysis. RNA was extracted from cells using an RNeasy minElute Clean-up Kit (Qiagen, Hilden, Germany) and quantified by Nanodrop Synergy HT (Biotek, Germany). The cDNA of each miRNA was synthesized by the respective Taqman miRNA Reverse Transcription Kit (Applied Biosystems, CA, USA).
Absolute quantification of miRNAs by Droplet Digital PCR
Absolute quantification of selected miRNAs was achieved using a BioRad droplet digital PCR (ddPCR). During the optimization experiment, Tm was determined as 60°C for miR-218-5p, miR-214-3p, and miR-140-3p and 57.4°C for miR-20a-5p and U6 depending upon the partition between positive and negative droplet clusters. Thermal cycling conditions for all assays were set at 95°C for 10 min (1 cycle), 94°C for 30 s and Tm-60°C/57.4°C for 60 s (40 cycles), 98°C for 10 min (ramp rate 1°C/s), and 4°C for 30 min and infinite hold at 12°C. Ramp rate of 1°C/s was set in every step. Briefly, 20 μL of the reaction mixture was prepared by addition of 10 μL of ddPCR Supermix for Probes (no dUTP) (1,863,010, (Bio-Rad Laboratories, CA, USA); 0.5 μL of TaqMan primer/probe mix specific for each miRNAs miR-218-5p (cat no. 000521), miR-214-3p (cat. no. 002306), miR-140-3p (cat. no. 002234), miR-20a-5p (cat. no. 000580), and U6 (cat no. 001973) (Thermo Fisher Scientific, USA); and 1 μL of cDNA sample with nuclease-free water and assay mix. The PCR reaction was loaded on the cartridge followed by the addition of 70 μL of Droplet Generation Oil (1,863,005, Bio-Rad Laboratories, CA, USA) in appropriate wells, and then droplets were generated using Droplet Generator QX200. Later, the droplets were transferred to a 96-well PCR plate (Eppendorf, Hamburg, Germany) and PCR was carried out in C1000 Touch Thermal Cycler (Bio-Rad Laboratories, CA, USA). After the amplification, the plate was loaded on QX200 Droplet Reader (Bio-Rad Laboratories, CA, USA) and both positive and negative droplets were read. In the end, QuantaSoft software, version 1.7.4 (QuantaSoft, Prague, Czech Republic) was used to calculate the absolute quantification of each miRNA. Further calculations were done as per the analysis described by Coulter et al. [27] and data was represented as relative quantification between controls and women with PCOS. To ensure experiment quality, wells having more than 14,000 total droplet counts were included in the analysis. Each sample was analyzed in duplicate.
Statistical analysis
Statistical analysis was used to compare miRNA expression data, hormonal, biochemical, and oocyte parameters between controls and PCOS by nonparametric Mann–Whitney U test methods. GraphPad Prism 9 software was used to carry out statistical analysis. The P < 0.05 was considered statistically significant.
Results
Differentially expressed angiogenic genes in the ovary of PCOS women
By literature search, a total of 112 studies that described angiogenesis in PCOS were retrieved. Eleven articles were included based on the selection criteria. These articles were screened and a list of 18 differentially expressed genes and proteins related to angiogenesis in the ovary of women with PCOS were identified.
In addition, seven expression studies on ovary/ovarian tissue/ovarian cells in PCOS vs controls, deposited in the GEO database, were retrieved and analyzed (Supplementary Table S1). Pathway enrichment of DEGs obtained from each of the seven datasets led to the identification of 87 genes associated with angiogenesis, FGF signaling, and VEGF signaling pathway. Thereafter, we combined the genes obtained by both approaches and got a final list of 99 unique target genes having a predicted role in angiogenesis in PCOS (“angiogenic dataset”) (Table 1). This is the first time that an integrated approach has been adopted to delineate angiogenic target genes which may have a role in PCOS pathophysiology.
Reported miRNAs in PCOS retrieved by literature search
MiRNAs reported in the ovary/ovarian tissues/follicular fluid in women with PCOS were retrieved by extensive literature search. Fifty-six articles were shortlisted from the 202 search results based on the selection criteria. The articles were mined to obtain a list of 874 miRNAs (“PCOS miRNA dataset”) reported to be expressed in the ovary of women with PCOS. Furthermore, 262 of these 874 miRNAs were reported to be dysregulated in PCOS by either high or low throughput studies (Supplementary Table S2). Of the 262 miRNAs, 81 were validated by low throughput studies.
MiRNAs regulating angiogenesis process retrieved from databases
We used miRNA databases like miRTarBase, and DIANA-TarBase that contain information on experimentally validated miRNA-mRNA interactions. The 99 unique genes in the “angiogenic dataset” were used to query the abovementioned miRNA databases. The search identified 428 unique miRNA-mRNA interaction pairs validated by low throughput studies which were taken for further analysis (Supplementary Table S3). The total of 218 unique miRNAs obtained from 428 unique miRNA-mRNA interaction pairs was then compared with the “PCOS miRNA dataset.” This analysis helped to identify 188 unique common miRNAs (Supplementary Table S4) regulating genes associated with angiogenesis and reported to be expressed in the ovary of women with PCOS. Functional enrichment analysis of these 188 miRNAs showed that they are enriched in angiogenesis-related pathways like PI3K/Akt signaling pathway, HIF-1 signaling pathway, VEGF signaling pathway, Notch signaling pathway, and extracellular matrix (ECM)-receptor interaction. Figure 2 represents the number of miRNAs present in each of these 5 pathways. The heatmap depicts the distribution of miRNAs in these pathways (Supplementary Fig. 1). The miRNAs (n = 111) common in at least 4 enriched angiogenesis pathways and not experimentally validated in PCOS earlier were shortlisted.
The number of miRNAs in enriched angiogenesis-associated pathway. Bar graph showing the number of miRNAs that are reported in PCOS and associated with different angiogenesis-related pathways by functional enrichment analysis. PI3K, phosphatidylinositol 3-kinase; HIF-1, hypoxia-inducing factor 1; VEGF, vascular endothelial growth factor; ECM, extracellular matrix
MiRNA selection for expression analysis
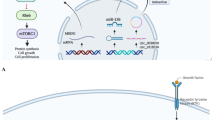
The PI3K/Akt pathway was found to be one of the major angiogenesis pathways with maximum gene and miRNA representation in the enrichment analysis (Figs. 2 and 3). Hence, miRNA-mRNA pairs identified to be involved in PI3K/Akt pathway based on pathway analysis were shortlisted. Four genes, namely VEGFA, fibroblast growth factor receptor 1 (FGFR1), PDGFRA, and fibronectin (FN1) from the PI3K-Akt pathway which have been reported to be dysregulated in PCOS and known to play a role in angiogenesis were selected. The miRNAs viz miR-218-5p, miR-214-3p, miR-20a-5p, and miR-140-3p specifically targeting these genes in the PI3K/Akt pathway were selected for further validation to understand their expression in GCs of PCOS and controls (Fig. 4).
Enriched angiogenesis-associated pathways of dysregulated target genes in PCOS. Network map depicts the dysregulated target genes in PCOS enriched in angiogenesis-related pathways. Pathway enrichment of differentially regulated angiogenesis-associated genes was carried out using KEGG pathway analysis in Cytoscape. Retrieved angiogenesis-associated pathways are represented in the figure
MiRNA-mRNA gene interaction network of four selected targets from the PI3K/Akt signaling pathway. The miRNA-mRNA interaction network was constructed using Cytoscape. The most enriched PI3K/Akt signaling pathway was used to select 4 angiogenesis-associated genes which were dysregulated in PCOS and 4 miRNAs regulating these genes. Purple-colored nodes represent miRNAs that are selected for validation by gene expression studies. PI3K, phosphatidylinositol 3-kinase
Study participant’s characteristics
The anthropometric, hormonal, and biochemical parameters of all the participants are outlined in Table 2. In the present study, 30 participants were enrolled, of which 15 were women with PCOS and 15 were healthy controls. The basal hormonal parameters, LH, LH:FSH ratios, and anti-mullerian hormone (AMH) were found to be significantly high while FSH levels were comparable between women with PCOS compared to controls. On the d-OPU, hormonal estimation showed significantly higher TT and androgen indices, namely, Free-T, Bio-T, and FAI levels and lower P4 and SHBG levels in the women with PCOS compared to controls in both serum and follicular fluid. Total oocytes and MII oocytes obtained from both groups were similar.
Absolute miRNA quantification
We performed ddPCR to quantitate the absolute concentration of selected miRNAs in GCs. All the four selected miRNAs were observed to be up-regulated, namely, miR-218-5p, miR-214-3p, miR-20a-5p, and miR-140-3p in PCOS (Fig. 5). Validated miRNAs with their all interacting genes from the “angiogenic dataset” were retrieved (Fig. 6). We observed that all these validated miRNAs target multiple angiogenic genes reported to be differentially altered in GCs of PCOS. This observation suggests that the altered expression of miRNAs and their interacting mRNA pair may be playing an essential role in the compromised angiogenic capacity of GCs in women with PCOS.
DDPCR analysis of miRNA expressions related to angiogenesis in granulosa cells from PCOS and controls. The graph represents the relative miRNA expressions in GCs of controls (n = 15) and women with PCOS (n = 15). Expression was normalized to the U6 miRNA as an endogenous control. Data are presented as “mean ± SEM” and *P < 0.05 was considered significant. Data are analyzed using the Mann–Whitney U test. DDPCR, digital droplet polymerase chain reaction
Interaction network of validated miRNAs and their reported target genes. The miRNA-mRNA network was constructed using Cytoscape. The miRNAs expression was validated in granulosa cells obtained from women with PCOS and controls. Targets are angiogenesis-associated genes that are reported to be differentially expressed in the ovary of women with PCOS compared. The green node and red node represent up and down-regulated target genes, respectively
Discussion
Angiogenesis is a complex process, involving the synchronous activity of diverse pro- and anti-angiogenic factors. Ovarian angiogenesis is vital for the growth and development of the follicles, oocyte, and CL formation. Evidence suggests a role of aberrant angiogenesis in the folliclar defect of women with PCOS; however, the mechanism has not yet been extensively explored. Identification of dysregulated angiogenesis-associated genes and their regulating miRNAs is crucial for understanding their role in the regulation of angiogenesis in PCOS. Hence, this study focused on the creation of a dataset of miRNA-mRNA pairs by integrating information on dysregulated genes with angiogenic potential and their regulating miRNAs expressed in the ovary of women with PCOS.
Studies have reported vascular alterations in the ovaries of women with PCOS which may affect supply of oxygen, nutrients, and hormones to the developing follicles and formation and functioning of CL. Few reports have also mentioned increased ovarian stromal vascularity in the PCOS ovary; however, the peri-follicular blood flow was observed to be unchanged or lower than the normal ovaries [8]. A hypoxic environment is the main driving force for blood vasculature formation. The coordinated action of VEGF and other angiogenic factors like ANGPT, FGF2, and PDGFs has been reported to be associated with an increase in vascular permeability, endothelial cell proliferation, and survival and maintenance of newly formed blood vessels. In the ovary of women with PCOS, various studies have reported differential expression of a few pro- and anti-angiogenic factors. Corroborating these findings, recent investigations have shown altered expression of angiogenic factors and decreased angiogenic capacity of GCs and follicular fluid in women with PCOS. All these existing pieces of evidence have indicated dysregulation of the angiogenesis process to be causal for follicular growth arrest, CL insufficiency, and frequent miscarriages observed in PCOS. Detailed information regarding the angiogenesis-associated genes which are altered in the ovary of women with PCOS will throw light on the dysregulated ovarian vasculature formation in these women.
Existing literature on “PCOS and angiogenesis” and the ovarian expression profiling datasets were mined from the PubMed and GEO databases, respectively. The ovarian expression profiling datasets were reanalyzed to obtain genes dysregulated in PCOS and functional enrichment of these genes led to the identification of a comprehensive list of 99 potential angiogenesis-associated genes in PCOS. This list includes ephrin and its receptors, VEGF and its receptors like KDR and Flt-1, Notch and Wnt signaling, Jagged-1, various members of FGF and PDGF families, several mitogen-activated protein kinases and focal adhesion kinases which play important role in blood vasculature formation. Hence, their altered expression in the ovary/ovarian tissues of women with PCOS indicates dysregulated angiogenesis in PCOS. To decipher the mechanism of dysregulated angiogenesis in PCOS, it is imperative to identify crucial regulators of gene expression, namely the miRNAs. Towards this, miRNAs reported to target these 99 dysregulated genes were fetched from miRNA databases and a miRNA-mRNA network was constructed. This gave rise to a list of candidate miRNA-mRNA pairs that may have a probable role in dysregulated angiogenesis in women with PCOS. Furthermore, miRNA-mRNA interactions validated by low throughput studies were used and unique predicted miRNAs were shortlisted. Simultaneously, we enlisted the miRNAs reported in the ovary of women with PCOS by literature search. We wanted to know how many of these miRNAs are expressed in the ovary of women with PCOS; therefore, we overlapped the predicted and reported miRNAs to obtain common miRNAs which possibly play a role in impaired follicular angiogenesis in PCOS. Pathway enrichment of this miRNA dataset generated in the study delineated PI3K/Akt pathway, VEGF and HIF-1 signaling pathway, ECM-receptor interaction, and Notch signaling pathway as the five enriched angiogenesis-associated pathways that may be altered in PCOS. The PI3K/Akt pathway was identified as the most prominent pathway associated with angiogenesis in PCOS.
To validate the in silico data, we selected miRNAs, miR-218-5p, miR-214-3p, miRNA-20a-5p, and miR-140-3p specifically targeting PDGFR1, FGFR1, VEGFA, and FN1 genes, respectively, in the PI3K/Akt pathway. The expression profile of the above four miRNAs was measured by ddPCR, a novel method allowing accurate absolute quantitation of miRNA transcripts. These target genes were selected based on evidence from existing literature on their association with angiogenesis. VEGFA and FGF2 are the most critical factors necessary for follicular vasculature formation. VEGFA increases vascular permeability and along with FGF2, helps in the sprouting and migration of endothelial cells during angiogenesis [3]. FGF2 binds with its receptor FGFR1 and mediates signaling reaction. Furthermore, ECM remodeling and basement degradation are essential for endothelial cell migration and proliferation during vasculature formation. FN1 is an important ECM-related factor that supports angiogenesis. The PDGF is involved in the maturation of the blood capillaries, whose signaling cascade is initiated by binding and dimerization of two related RTKs receptors, PDGFA- or B-receptors. Recent reports have shown a down-regulation of FGFR1, VEGFA, and FN1 genes in GCs of women with PCOS [3, 4]. Scotti et al. reported lower levels of PDGFB in follicular fluid of women with PCOS [28].
The expression of all four miRNAs, miR-218-5p, miR-214-3p, miRNA-20a-5p, and miR-140-3p were up-regulated in GCs of women with PCOS and controls. Predicted targets of miR-218-5p and miR-214-3p from the “angiogenic dataset” and from available literature are reported to negatively regulate angiogenesis in tumors [29, 30]. The increased levels of miR-218-5p and miR-214-3p were demonstrated to suppress pro-angiogenic genes expression such as PDGFRA, FN1, HIF, VEGFA, ANGPT-1 and 2, matrix metallo protein-2, PDGF, PDGFβR, FGF2, and FGFR1 [31,32,33,34,35,36,37,38]. Our microarray data analysis and the literature search revealed that the miR-20a-5p binds to several pro-angiogenic genes like VEGFA, HIF1A, FGF2, ADAM metallopeptidase with thrombospondin type 1 motif 1 (ADAMTS1), integrin subunit alpha 5, transforming growth factor beta-1, and tissue inhibitor of metalloproteinase and suppresses their expression and thus exerts anti-angiogenic activity [39]. Interestingly, down-regulation of FN1, HIF1A, VEGFA, ANGPT-1, FGF2, and FGFR1 have been reported in GCs of women with PCOS which are the targets of these miRNAs [3, 4]. This explains one of the reasons for the down-regulation of these genes could be overexpression of miR-218-5p, miR-214- 3p, and miR-20a-5p in PCOS. The miR-140-3p has been reported to inhibit the expression of ECM genes like FN1 and heparan sulfate proteoglycan 2 (HSPG2) which play a pivotal role in angiogenesis [40, 41]. We observed increased miR-140-3p expression in PCOS, interestingly its target genes FN1 and HSPG2 were down-regulated in GCs of women with PCOS as reported by us previously, indicating miR-140-3p may be responsible for decreased expression of these targets in PCOS [3, 4]. Therefore, current findings strongly suggest that these miRNAs may be playing a key role in the altered expression of angiogenesis-related genes in GCs of women with PCOS. Hence, our findings on angiogenesis-related miRNA-mRNA interactions in the ovary of women with PCOS may prove a valuable resource to study angiogenesis in PCOS in detail which partly contributes to its pathophysiology.
The limitation of this study is the low sample size due to poor quality and/or quantity of GCs, exclusion of participants based on selection criteria, and dearth of samples due to an ongoing pandemic. However, the experiments were conclusive for validating the in-silico data used to identify predicted angiogenesis-associated miRNA-mRNA pairs.
Conclusion
Altogether, this study was successful in identifying and generating datasets of potential angiogenesis-associated target genes and their regulating miRNAs. We also identified angiogenesis-associated pathways which are majorly affected in PCOS. The datasets and findings of this study will prove to be an important resource for researchers interested in unraveling the mechanism of altered angiogenesis in PCOS ovary and also to augment future research on possible therapeutic options. In our view, this is an innovative analysis strategy that may help to shed light on the molecular mechanism of altered angiogenesis in PCOS which may contribute to defect in folliculogenesis and compromised formation and functioning of CL leading to luteal phase insufficiency and frequent miscarriages in these women. Furthermore, experimental and functional studies on a specific miRNA against angiogenic targets are needed to evaluate its role in the regulation of angiogenesis.
Availability of data and material
All data is included either in manuscript or in supplemental files.
References
Sagvekar P, Dadachanji R, Patil K, Mukherjee S. Pathomechanisms of polycystic ovary syndrome: multidimensional approaches. Front Biosci Elit. 2018;10:384–422.
Sagvekar P, Kumar P, Mangoli V, Desai S, Mukherjee S. DNA methylome profiling of granulosa cells reveals altered methylation in genes regulating vital ovarian functions in polycystic ovary syndrome. Clin Epigenetics. 2019;11:1–16.
Patil K, Hinduja I, Mukherjee S. Alteration in angiogenic potential of granulosa-lutein cells and follicular fluid contributes to luteal defects in polycystic ovary syndrome. Hum Reprod. 2021;36:1052–64.
Ambekar AS, Kelkar DS, Pinto SM, Sharma R, Hinduja I, Zaveri K, et al. Proteomics of follicular fluid from women with polycystic ovary syndrome suggests molecular defects in follicular development. J Clin Endocrinol Metab. 2015;100:744–53.
Tamanini C, De Ambrogi M. Angiogenesis in developing follicle and corpus luteum. Reprod Domest Anim. 2004;39:206–16.
Sagle M, Bishop K, Ridley N, Alexander FM, Michel M, Bonney RC, et al. Recurrent early miscarriage and polycystic ovaries. Br Med J. 1988;297:1027–8.
Meenakumari KJ, Agarwal S, Krishna A, Pandey LK. Effects of metformin treatment on luteal phase progesterone concentration in polycystic ovary syndrome. Braz J Med Biol Res. 2004;37:1637–44.
Tal R, Seifer DB, Arici A. The emerging role of angiogenic factor dysregulation in the pathogenesis of polycystic ovarian syndrome. Semin Reprod Med. 2015;33:195–207.
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:1–12.
Hossain M, Ghanem N, Hoelker M, Rings F, Phatsara C, Tholen E, et al. Identification and characterization of miRNAs expressed in the bovine ovary. BMC Genomics. 2009;10:443.
Wu F, Yang Z, Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun. Elsevier Inc.; 2009;386:549–53. Available from: https://doi.org/10.1016/j.bbrc.2009.06.075.
Da Silveira JC, Winger QA, Bouma GJ, Carnevale EM. Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-β signalling during follicle development in the mare. Reprod Fertil Dev. 2015;27:897–905.
McGinnis LK, Luense LJ, Christenson LK. MicroRNA in ovarian biology and disease. Cold Spring Harb Perspect Med. 2015;5:1–20.
Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, et al. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118:2366. https://doi.org/10.1172/JCI33680.
Alexandri C, Daniel A, Bruylants G, Demeestere I. The role of microRNAs in ovarian function and the transition toward novel therapeutic strategies in fertility preservation: from bench to future clinical application. Hum Reprod Update. 2020;26:174–96.
Agarwala R, Barrett T, Beck J, Benson DA, Bollin C, Bolton E, et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res Oxford University Press. 2018;46:D8-13.
Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, et al. NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res. 2009;37:885–90.
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Sel Work Terry Speed. 2012;601–16.
Wettenhall JM, Smyth GK. limmaGUI: a graphical user interface for linear modeling of microarray data. Bioinformatics. 2004;20:3705–6.
Huang HY, Lin YCD, Li J, Huang KY, Shrestha S, Hong HC, et al. MiRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res Oxford University Press. 2020;48:D148–54.
Karagkouni D, Paraskevopoulou MD, Chatzopoulos S, Vlachos IS, Tastsoglou S, Kanellos I, et al. DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res Oxford University Press. 2018;46:D239–45.
Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res Oxford University Press. 2021;49:D605–12.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Rotterdam. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. United States; 2004;81:19–25.
Patil K, Yelamanchi S, Kumar M, Hinduja I, Prasad TSK, Gowda H, et al. Quantitative mass spectrometric analysis to unravel glycoproteomic signature of follicular fluid in women with polycystic ovary syndrome. PLoS One. 2019;14:e0214742.
Mukherjee S, Shaikh N, Khavale S, Shinde G, Meherji P, Shah N, et al. Genetic variation in exon 17 of INSR is associated with insulin resistance and hyperandrogenemia among lean Indian women with polycystic ovary syndrome. Eur J Endocrinol England. 2009;160:855–62.
Coulter SJ. Mitigation of the effect of variability in digital PCR assays through use of duplexed reference assays for normalization. Biotechniques. 2018;65:86–91.
Scotti L, Parborell F, Irusta G, De Zuñiga I, Bisioli C, Pettorossi H, et al. Platelet-derived growth factor BB and DD and angiopoietin1 are altered in follicular fluid from polycystic ovary syndrome patients. Mol Reprod Dev. 2014;81:748–56.
Xiangyuan Z, Jiaqiang D, Yan H, Ming Z, Zhen L, Na W, et al. miR-218 inhibited tumor angiogenesis by targeting ROBO1 in gastric cancer. Gene. Elsevier B.V.; 2017;615:42–9. Available from: https://doi.org/10.1016/j.gene.2017.03.022.
Balkom BWMva, Jong OGd, Smits M, Brummelman J, den Ouden K, Bree PMd, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006.
Bobbs AS, Saarela AV, Yatskievych TA, Antin PB. Fibroblast Growth Factor (FGF) signaling during gastrulation negatively modulates the abundance of microRNAs that regulate proteins required for cell migration and embryo patterning. J Biol Chem. 2012;287:38505–14.
Tang S, Wang D, Zhang Q, Li L. miR-218 suppresses gastric cancer cell proliferation and invasion via regulation of angiopoietin-2. Exp Ther Med. 2016;12:3837–42.
Lun W, Wu X, Deng Q, Zhi F. MiR-218 regulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via targeting CTGF. Cancer Cell Int. BioMed Central; 2018;18:1–9. Available from: https://doi.org/10.1186/s12935-018-0575-2.
Guan B, Wu K, Zeng J, Xu S, Mu L, Gao Y, et al. Tumor-suppressive microRNA-218 inhibits tumor angiogenesis via targeting the mTOR component RICTOR in prostate cancer. Oncotarget. 2017;8:8162–72.
Yang Y, Li Z, Yuan H, Ji W, Wang K, Lu T, et al. Reciprocal regulatory mechanism between miR-214–3p and FGFR1 in FGFR1-amplified lung cancer. Oncogenesis. Springer US; 2019;8:1–15. Available from: https://doi.org/10.1038/s41389-019-0151-1.
Yahya SMM, Yahya SMM. The Effect of miR-98 and miR-214 on Apoptotic and angiogenic pathways in hepatocellular carcinoma HepG2 cells. Indian J Clin Biochem. Springer India; 2020;35:353–8. Available from: https://doi.org/10.1007/s12291-019-00824-1.
Van Mil A, Grundmann S, Goumans MJ, Lei Z, Oerlemans MI, Jaksani S, et al. MicroRNA-214 inhibits angiogenesis by targeting quaking and reducing angiogenic growth factor release. Cardiovasc Res. 2012;93:655–65.
Li Y, Zhao L, Qi Y, Yang X. MicroRNA-214 upregulates HIF-1α and VEGF by targeting ING4 in lung cancer cells. Mol Med Rep Greece. 2019;19:4935–45.
Xiong X, Sun Y, Wang X. HIF1A/miR-20a-5p/TGFβ1 axis modulates adipose-derived stem cells in a paracrine manner to affect the angiogenesis of human dermal microvascular endothelial cells. J Cell Physiol. 2020;235:2091–101.
Duru N, Zhang Y, Gernapudi R, Wolfson B, Lo PK, Yao Y, et al. Loss of miR-140 is a key risk factor for radiation-induced lung fibrosis through reprogramming fibroblasts and macrophages. Sci Rep. Nature Publishing Group; 2016;6:1–12. Available from: https://doi.org/10.1038/srep39572.
Haecker I, Gay LA, Yang Y, Hu J, Morse AM, McIntyre LM, et al. Ago HITS-CLIP expands understanding of Kaposi’s sarcoma-associated herpesvirus miRNA function in primary effusion lymphomas. PLoS Pathog. 2012;8:e1002884.
Acknowledgements
The authors would like to express gratitude to all recruited study participants. We are thankful to Ms. Hima Shah (Mumbai Fertility Clinic & IVF Centre, Mumbai, India) for collecting samples and providing access to clinical records of participants. The authors acknowledge support from the Indian Council of Medical Research-National Institute for Research in Reproductive Health, Mumbai, India (ICMR-NIRRH) (RA/1112/08-2021). We would like to thank the financial assistance provided by ICMR, Government of India to KP for pursuing her doctoral studies.
Funding
This work was supported by Grant BT/PR16524/MED/97/346/2016 from the Department of Biotechnology, Government of India.
Author information
Authors and Affiliations
Contributions
KP: designed and carried out in-silico analysis, experiments, analyzed the results, and drafted the manuscript. SJ: guided with in-silico analysis and revised the manuscript.SM: concept, design, supervised and approved manuscript for submission. JS: provided clinical samples and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was conducted at ICMR-National Institute for Research in Reproductive Health (ICMR-NIRRH), Mumbai, India after obtaining ethical permission (Ethical approval no: 283/2015).
Consent to participate
All study participants provided signed informed consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, K., Joseph, S., Shah, J. et al. An integrated in silico analysis highlighted angiogenesis regulating miRNA-mRNA network in PCOS pathophysiology. J Assist Reprod Genet 39, 427–440 (2022). https://doi.org/10.1007/s10815-022-02396-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02396-1