Abstract
Purpose
To investigate whether the ability of human spermatozoa to decondense in vitro in the presence of heparin (Hep) and glutathione (GSH) is related to assisted reproduction (ART) success.
Methods
Cross-sectional pilot study involving male partners of 129 infertile couples undergoing ICSI with (45) or without (84) donor oocytes at two infertility clinics in CABA, Argentina, between October 2012 and December 2013. In vitro decondensation kinetics with Hep and GSH and DNA fragmentation (TUNEL) were determined on the same sample used for ICSI. The possible relationship of decondensation parameters (maximum decondensation and decondensation velocity) and TUNEL values with ART success was evaluated.
Results
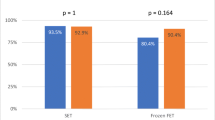
Embryo quality correlated positively with decondensation velocity (D60/D30) (Spearman’s correlation, p < 0.05). According to D60/D30 values, patients were classified as slow decondensers (SlowD) (n = 68) or fast decondensers (FastD) (n = 61). Embryo quality was better in FastD (unpaired t test, p < 0.05). FastD and SlowD were subdivided according to use of donor oocytes. Among SlowD, biochemical and clinical pregnancy rates per transfer were significantly higher in donor (n = 19) vs. in non-donor (n = 31) cycles (Fisher’s exact test, p < 0.05). TUNEL values were not related to embryo quality, but no clinical pregnancies or live births were achieved in TUNEL+ SlowD (n = 7).
Conclusion
Decondensation kinetics of human spermatozoa in vitro with Hep and GSH could be related to embryo quality and ART success.







Similar content being viewed by others
References
Esterhuizen AD, Franken DR, Becker PJ, Lourens JGH, Muller II, Van Rooyen LH. Defective sperm decondensation: a cause for fertilization failure. Andrologia. 2002;34:1–7.
Razavi S, Nasr-Esfahani MH, Mardani M, Mafi A, Moghdam A. Effect of human sperm chromatin anomalies on fertilization outcome post-ICSI. Andrologia. 2003;35:238–43.
Hamamah S, Fignon A, Lansac J. The effect of male factors in repeated spontaneous abortion: lesson from in vitro fertilization and intracytoplasmic sperm injection. Hum Reprod Update. 1997;3:393–400.
Saxena P, Misro MM, Chaki SP, Chopra K, Roy S, Nandan D. Is abnormal sperm function an indicator among couples with recurrent pregnancy loss? Fertil Steril. 2008;90:1854–8.
Steger K, Wilhelm J, Konrad L, Satlf T, Greb R, Diemer T, et al. Both protamine-1 to protamine-2 mRNA ratio and Bcl2 mRNA content in testicular spermatids and ejaculated spermatozoa discriminate between fertile and infertile men. Hum Reprod. 2008;23:11–6.
Boulet SL, Mehta A, Kissin D, Warner L, Kawwass JF, Jamieson DJ. Trends in use of and reproductive outcomes associated with intracytoplasmic sperm injection. JAMA. 2015;313:255–63.
Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, et al. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod BioMed Online. 2003;7:477–84.
Muriel L, Garrido N, Fernández JL, Remohí J, Pellicer A, de los Santos MJ, et al. Value of the sperm deoxyribonucleic acid fragmentation level, as measured by the sperm chromatin dispersion test, in the outcome of in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2006;85:371–83.
Lin MH, Lee RKK, Li SH, Lu CH, Sun FJ, Hwu YM. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection but might be related to spontaneous abortion rates. Fertil Steril. 2008;90:352–9.
Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–31.
Tavalaee M, Razavi S, Nasr-Esfahani MH. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91:1119–26.
Tarozzi N, Nadalini M, Stronati A, Bizzaro D, Dal Prato L, Coticchio G, et al. Anomalies in sperm chromatin packaging: implications for assisted reproduction techniques. Reprod BioMed Online. 2009;18:486–95.
Palermo GD, Neri QV, Cozzubbo T, Rosenwaks Z. Perspectives on the assessment of human sperm chromatin integrity. Fertil Steril. 2014;102:1508–17.
Romanato M, Regueira E, Cameo MS, Baldini C, Calvo L, Calvo JC. Further evidence on the role of heparan sulfate as protamine acceptor during the decondensation of human spermatozoa. Hum Reprod. 2005;20:2784–9.
Romanato M, Julianelli V, Zappi M, Calvo L, Calvo JC. The presence of heparan sulfate in the mammalian oocyte provides a clue to human sperm nuclear decondensation in vivo. Hum Reprod. 2008;23:1145–50.
Julianelli VL, Farrando B, Alvarez Sedó C, Calvo L, Romanato M, Calvo JC. Heparin enhances protamine disulfide bond reduction during in vitro decondensation of human spermatozoa. Hum Reprod. 2012;27:1930–8.
Sanchez MC, Sedo CA, Julianelli VL, Romanato M, Calvo L, Calvo JC, et al. Dermatan sulfate synergizes with heparin in murine sperm chromatin decondensation. Syst Biol Reprod Med. 2013;59:82–90.
Perreault SD. Regulation of sperm nuclear reactivation during fertilization. In Bavister BD, Cummins J, Roldan ERS (eds). Fertilization in Mammals. Norwell: Serono Symposia USA; 1990. pp 285–296.
Canel NG, Bevacqua RJ, Hiriart MI, Rabelo NC, de Almeida Camargo LS, Romanato M, et al. Sperm pretreatment with heparin and L-glutathione, sex-sorting, and double cryopreservation to improve intracytoplasmic sperm injection in bovine. Theriogenology. 2017;93:62–70.
Gaubeca-Klix E, Marin-Briggiler C, Cameo M and Calvo L. In vitro sperm nuclear decondensation in the presence of heparin/glutathione distinguishes two subgroups of infertile patients not identified by conventional semen analysis. In: Treatment of infertility: the new Frontiers (abstract book). Boca Raton, FL, 1998: Abstract PO-51.
Bergere M, Selva J, Baud M, Volante M, Martin B, Hugues JN, et al. Chromosome 18 analysis by fluorescence in situ hybridization (FISH) in human blastomeres of abnormal embryos after in vitro fertilization (IVF) attempt. Prenat Diagn. 1995;15:835–41.
WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO Press, World Health Organization; 2010.
Romanato M, Cameo MS, Bertolesi G, Baldini C, Calvo JC, Calvo L. Heparan sulphate: a putative decondensing agent for human spermatozoa in vivo. Hum Reprod. 2003;18:1868–73.
Reyes R, Rosado A, Hernández O, Delgado NM. Heparin and glutathione: physiological decondensing agents of human sperm nuclei. Gamete Res. 1989;23:39–47.
Bedford JM, Bent MJ, Calvin H. Variations in the structural character and stability of the nuclear chromatin in morphologically normal human spermatozoa. J Reprod Fertil. 1973;33:19–29.
Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8.
Brown DB, Merryman DC, Rivnay B, Houserman VL, Long CA, Honea KL. Evaluating a novel panel of sperm function tests for utility in predicting intracytoplasmic sperm injection (ICSI) outcome. J Assist Reprod Genet. 2013;30:461–77.
Kavitha P, Malini SS. Positive association of sperm dysfunction in the pathogenesis of recurrent pregnancy loss. J Clin Diagn Res. 2014;8:OC07–10.
Barratt CLR, De Jonge CJ, Sharpe R. Man up’: the importance and strategy for placing male reproductive health Centre stage in the political and research agenda. Hum Reprod. 2018;33(4):541–5.
D'Occhio MJ, Hengstberger KJ, Johnston SD. Biology of sperm chromatin structure and relationship to male fertility and embryonic survival. Anim Reprod Sci. 2007;101:1–7.
Erenpreiss J, Spano M, Erenpreisa J, Bungum M, Giwercman A. Sperm chromatin structure and male fertility: biological and clinical aspects. Asian J Androl. 2006;8:11–29.
Evgeni E, Charalabopoulos K, Asimakopoulos B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J Reprod Infertil. 2014;15:2–14.
Menezo Y, Clement P, Amar E. Evaluation of sperm DNA structure, fragmentation and decondensation: an essential tool in the assessment of male infertility. Transl Androl Urol. 2017;6:S553–6.
Cebesoy FB, Aydos K, Unlu C. Effect of sperm chromatin damage on fertilization ratio and embryo quality post-ICSI. Arch Androl. 2006;52:397–402.
Depa-Martynow M, Kempistry B, Lianeri M, Jagodizinski PP, Jedrzejczak P. Association between fertilin B, protamines 1 and 2 and sperm specific linker histone H-1 like protein mRNA levels, fertilization ability of human spermatozoa and the quality of preimplantation embryos. Folia Histochem Cytobiol. 2007;45:79–85.
Kazerooni T, Asadi N, Jadid L, Kazerooni M, Ghanadi A, Ghaffarpasand F, et al. Evaluation of sperm’s chromatin quality with acridine orange test, chromomycin A3 and aniline blue staining in couples with unexplained recurrent abortion. J Assist Reprod Genet. 2009;26:591–6.
Irez T, Sahmay S, Ocal P, Goymen A, Senol H, Erol N, et al. Investigation of the association between the outcomes of sperm chromatin condensation and decondensation tests, and assisted reproduction techniques. Andrologia. 2015;47:438–47.
Meseguer M, Santiso R, Garrido N, García-Herrero S, Remohí J, Fernandez JL. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95:124–8.
Jin J, Pan C, Fei Q, Ni W, Yang X, Zhang L, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril. 2015;103:910–6.
Karydis S, Asimakopoulos B, Papadopoulos N, Vakalopoulos I, Al-Hasani S, Nicolettos N. ICSI outcome is not associated with the incidence of spermatozoa with abnormal chromatin condensation. In Vivo. 2005;19:921–5.
Simon L, Emery BR, Carrell DT. Review: diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Practice & Research Clinical Obstetrics and Gynaecology. 2017;44:38–56.
Funding
This work was supported by CONICET grant BID PICT 2012-2489 and by a grant from Fundación Bigand, Buenos Aires, Argentina. MYC and CG are doctoral fellows, CONICET, Argentina.
Author information
Authors and Affiliations
Contributions
CG, MYC, and VJ contributed substantially to acquisition, analysis, and interpretation of data corresponding to in vitro sperm decondensation. RR and LR contributed substantially to acquisition, analysis, and interpretation of data corresponding to DNA fragmentation assessment and ART performance. GRV supervised all clinical procedures. GRV, LC, JCC, VJ, and MR contributed substantially to conception and design, data analysis, and interpretation of data and drafting of the article. All authors contributed substantially to drafting and critical revision of the article and approved the final version submitted for publication.
Corresponding author
Ethics declarations
This study was approved by CEPI (Ethics Committee of Hospital Italiano de Buenos Aires) and IBYME Ethics Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galotto, C., Cambiasso, M.Y., Julianelli, V.L. et al. Human sperm decondensation in vitro is related to cleavage rate and embryo quality in IVF. J Assist Reprod Genet 36, 2345–2355 (2019). https://doi.org/10.1007/s10815-019-01590-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01590-y