Abstract
Purpose
Our purpose was to study whether application of femtosecond laser pulses for alphanumeric code marking in the volume of zona pellucida (ZP) could be effective and reliable approach for direct tagging of preimplantation embryos.
Methods
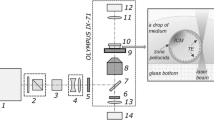
Femtosecond laser pulses (wavelength of 514 nm, pulse duration of 280 fs, repetition rate of 2.5 kHz, pulse energy of 20 nJ) were applied for precise alphanumeric code engraving on the ZP of mouse embryos at the zygote stage for individual embryo marking and their accurate identification. Embryo quality assessment every 24 h post laser-assisted marking as well as immunofluorescence staining (for ICM/TE cell number ratio calculation) were performed.
Results
Initial experiments have started with embryo marking in a single equatorial plane. The codes engraved could be clearly recognized until the thinning of the ZP prior to hatching. Since embryo may change its orientation during the ART cycle, multi-plane code engraving seems to be more practical for simplifying the process of code searching and embryo identification. We have marked the ZP in three planes, and no decrease in developmental rates as well as no morphological changes of embryos post laser-assisted engraving have been observed as compared to control group embryos.
Conclusions
Our results demonstrate the suitability of femtosecond laser as a novel tool for noninvasive embryo tagging, enabling embryo identification from day 0.5 post coitum to at least early blastocyst stage. Thus, the versatility and the potential use of femtosecond lasers in the field of developmental biology and assisted reproduction have been shown.







Similar content being viewed by others
References
Bedient C, Khanna P, Desai N. Laser pulse application in IVF. In: Jakubczak K, editor. Lasers - applications in science and industry: InTech; 2011. p. 193–214.
Karu TI. Lasers in infertility treatment: irradiation of oocytes and spermatozoa. Photomed Laser Surg. 2012;30:239–41. https://doi.org/10.1089/pho.2012.9888.
Abdel-Salam Z, Harith MA. Laser researches on livestock semen and oocytes: a brief review. J Adv Res. 2015;6:311–7. https://doi.org/10.1016/j.jare.2014.11.006.
Montag M, Rink K, Delacretaz G, van der Ven H. Laser induced immobilization and plasma membrane permeabilization in human spermatozoa. Hum Reprod. 2000;15:846–52. https://doi.org/10.1093/humrep/15.4.846.
Sato H, Landthaler M, Haina D, Schill WB. The effects of laser light on sperm motility and velocity in vitro. Andrologia. 1984;16:23–5.
Lenzi A, Claroni F, Gandini L, Lombardo F, Barbieri C, Lino A, et al. Laser radiation and motility patterns of human sperm. Syst Biol Reprod Med. 1989;23:229–34.
Preece D, Chow KW, Gomez-Godinez V, Gustafson K, Esener S, Ravida N, et al. Red light improves spermatozoa motility and does not induce oxidative DNA damage. Sci Rep. 2017;7:46480. https://doi.org/10.1038/srep46480.
Zhang H, Liu KK. Optical tweezers for single cells. J R Soc Interface. 2008;5:671–90. https://doi.org/10.1098/rsif.2008.0052.
Nascimento JM, Shi LZ, Meyers S, Gagneux P, Loskutoff NM, Botvinick EL, et al. The use of optical tweezers to study sperm competition and motility in primates. J R Soc Interface. 2008;5:297–302. https://doi.org/10.1098/rsif.2007.1118.
Clement-Sengewald A, Schütze K, Ashkin A, Palma GA, Kerlen G, Brem G. Fertilization of bovine oocytes induced solely with combined laser microbeam and optical tweezers. J Assist Reprod Genet. 1996;13:259–65. https://doi.org/10.1007/BF02065947.
Clement-Sengewald A, Buchholz T, Schütze K, Berg U, Berg FD. Noncontact, laser-mediated extraction of polar bodies for prefertilization genetic diagnosis. J Assist Reprod Genet. 2002;19:183–94. https://doi.org/10.1023/A:1014894029099.
Ilina IV, Rakityanskiy MM, Sitnikov DS, Ovchinnikov AV, Agranat MB, Khramova YV, et al. Biomedical and biotechnology applications of noncontact femtosecond laser microsurgery of living cells. AIP Conf Proc. 2012;1464:560–71. https://doi.org/10.1063/1.4739909.
Douglas-Hamilton DH, Conia J. Thermal effects in laser-assisted pre-embryo zona drilling. J Biomed Opt. 2001;6:205–13.
Tucker MJ, Ball GD. Assisted hatching as a technique for use in human in vitro fertilization and embryo transfer is long overdue for careful and appropriate study. J Clin Embr. 2009;12:5–8.
Taylor T, Gilchrist J, Hallowell S, Hanshew K, Orris J, Glassner M, et al. The effects of different laser pulse lengths on the embryo biopsy procedure and embryo development to the blastocyst stage. J Assist Reprod Genet. 2010;27:663–7. https://doi.org/10.1007/s10815-010-9461-0.
Stevenson DJ, Gunn-Moore FJ, Campbell P, Dholakia K. Single cell optical transfection. J R Soc Interface. 2010;7:863–71. https://doi.org/10.1098/rsif.2009.0463.
Torres-Mapa ML, Antkowiak M, Cizmarova H, Ferrier DEK, Dholakia K, Gunn-Moore FJ. Integrated holographic system for all-optical manipulation of developing embryos. Biomed Opt Express. 2011;2:1564–75. https://doi.org/10.1364/BOE.2.001564.
Ilina IV, Ovchinnikov AV, Sitnikov DS, Rakityanskiy MM, Agranat MB, Khramova YV, et al. Application of femtosecond laser pulses in biomedical cell technologies. High Temp. 2013;51:173–8. https://doi.org/10.1134/S0018151X13020089.
Osychenko AA, Zalessky AD, Krivokharchenko AS, Shakhbazian AK, Ryabova AV, Nadtochenko VA. Fusion of blastomeres in mouse embryos under the action of femtosecond laser radiation. Efficiency of blastocyst formation and embryo development. Quantum Electron. 2015;45:498–502. https://doi.org/10.1070/QE2015v045n05ABEH015767.
Kuetemeyer K, Lucas-Hahn A, Petersen B, Lemme E, Hassel P, Niemann H, et al. Combined multiphoton imaging and automated functional enucleation of porcine oocytes using femtosecond laser pulses. J Biomed Opt. 2010;15:046006. https://doi.org/10.1117/1.3463012.
Ilina IV, Khramova YV, Filatov MA, Semenova ML, Sitnikov DS. Application of femtosecond laser scalpel and optical tweezers for noncontact biopsy of late preimplantation embryos. High Temp. 2015;53:804–9. https://doi.org/10.1134/S0018151X15060103.
Liebler R. Are you my parent? Are you my child? The role of genetics and race in defining relationships after reproductive technological mistakes. DePaul J Health Care L. 2002;5:15–56.
Spriggs M. 2003 IVF mixup: white couple have black babies. J Med Ethics. 2003;29:65. https://doi.org/10.1136/jme.29.2.65.
Bender L. ‘To err is human’. ART mix-ups: a labor-based, relational proposal. J Gender Race & Just. 2006;9:1–90.
Forte M, Faustini F, Maggiulli R, Scarica C, Romano S, Ottolini C, et al. 2016 electronic witness system in IVF—patients perspective. J Assist Reprod Genet. 2016;33:1215–22. https://doi.org/10.1007/s10815-016-0759-4.
de los Santos MJ, Ruiz A. Protocols for tracking and witnessing samples and patients in assisted reproductive technology. Fertil Steril. 2013;100:1499–502. https://doi.org/10.1016/j.fertnstert.2013.09.029.
The Practice Committe of the American Society for Reproductive Medicine and the Practice Committe of the Society for Assisted Reproductive Technology. Revised guidelines for human embryology and andrology laboratories. Fertil Steril. 2008;90:S45–59. https://doi.org/10.1016/j.fertnstert.2008.08.099.
Glew AM, Hoha K, Graves J, Lawrence H, Read S, Ah-Moye M. Radio frequency identity tags ‘RFID’ for electronic witnessing of IVF laboratory procedures. Fertil Steril. 2006;86:S170. https://doi.org/10.1016/j.fertnstert.2006.07.454.
Thornhill AR, Brunetti XO, Bird S. Measuring human error in the IVF laboratory using an electronic witnessing system // Proc. of 17th World Congress on Controversies in Obstetrics, Gynecology & Infertility (COGI). 2013;101–106.
Schnauffer K, Kingsland C, Troup S. Barcode labelling in the IVF laboratory. Hum Reprod. 2005;20(suppl.1):i79–80.
Novo S, Nogues C, Penon O, Barrios L, Santalo J, Gomez-Martinez R, et al. Barcode tagging of human oocytes and embryos to prevent mix-ups in assisted reproduction technologies. Hum Reprod. 2014;29:18–28. https://doi.org/10.1093/humrep/det409.
Hur YS, Ryu EK, Park SJ, Yoon J, Yoon SH, Yang GD, et al. Development of a security system for assisted reproductive technology (ART). J Assist Reprod Genet. 2014;32:155–68. https://doi.org/10.1007/s10815-014-0367-0.
Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. New York: Cold Spring Harbor Lab; 2014.
Yan Z, Liang H, Deng L, Long H, Chen H, Chai W, et al. Eight-shaped hatching increases the risk of inner cell mass splitting in extended mouse embryo culture. PLoS One. 2015;10:e0145172. https://doi.org/10.1371/journal.pone.0145172.
Germond M, Nocera D, Senn A, Rink K, Delacrétaz G, Fakan S. Microdissection of mouse and human zona pellucida using a 1.48-microns diode laser beam: efficacy and safety of the procedure. Fertil Steril. 1995;64:604–11.
Hsieh YY, Huang CC, Cheng TC, Chang CC, Tsai HD, Lee MS. Laser-assisted hatching of embryos is better than the chemical method for enhancing the pregnancy rate in women with advanced age. Fertil Steril. 2002;78:179–82.
Malter HE, Schimmel T, Cohen J. Zona dissection by infrared laser: developmental consequences in the mouse, technical considerations, and controlled clinical trial. Reprod BioMed Online. 2001;3:117–23.
Fedele D, Fusi F. Thermal effects of NIR laser radiation in biological tissue: a brief survey. Energy for Health. 2010;6:10–5.
Sagoskin AW, Han T, Graham JR, Levy MJ, Stillman RJ, Tucker MJ. Healthy twin delivery after day 7 blastocyst transfer coupled with assisted hatching. Fertil Steril. 2002;77:615–7.
Li MW, Kinchen KL, Vallelunga JM, Young DL, Wright KDK, Gorano LN, et al. Safety, efficacy and efficiency of laser-assisted IVF in subfertile mutant mouse strains. Reproduction. 2013;145:245–54. https://doi.org/10.1530/REP-12-0477.
Anzai M, Nishiwaki M, Yanagi M, Nakashima T, Kaneko T, Taguchi Y, et al. Application of laser-assisted zona drilling to in vitro fertilization of cryopreserved mouse oocytes with spermatozoa from a subfertile transgenic mouse. J Reprod Dev. 2006;52:601–6.
Karmenyan AV, Shakhbazyan AK, Sviridova-Chailakhyan TA, Krivokharchenko AS, Chiou AE, Chailakhyan LM. Use of picosecond infrared laser for micromanipulation of early mammalian embryos. Mol Reprod Dev. 2009;76:975–83. https://doi.org/10.1002/mrd.21045.
Vogel A, Noack J, Huttman G, Paltauf G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl Phys B Lasers Opt. 2005;81:1015–47. https://doi.org/10.1007/s00340-005-2036-6.
Loesel FH, Fischer JP, Götz MH, Horvath C, Juhasz T, Noack F, et al. Non-thermal ablation of neural tissue with femtosecond laser pulses. Appl Phys B Lasers Opt. 1998;66:121–8.
Suhm N, Gotz MH, Fischer JP, Loesel F, Schlegel W, Sturm V, et al. Ablation of neural tissue by short-pulsed lasers–a technical report. Acta Neurochir. 1996;138:346–9. https://doi.org/10.1007/BF01411747.
Juhasz T, Kastis GA, Suarez C, Bor Z, Bron WE. Time-resolved observations of shock waves and cavitation bubbles generated by femtosecond laser pulses in corneal tissue and water. Lasers Surg Med. 1996;19:23–31. https://doi.org/10.1002/(SICI)1096-9101(1996)19:1<23::AID-LSM4>3.0.CO;2-S.
Oraevsky AA, Da Silva LB, Rubenchik AM, Feit MD, Glinsky ME, Perry MD, et al. 1996 plasma mediated ablation of biological tissues with nanosecond-to-femtosecond laser pulses: relative role of linear and nonlinear absorption. IEEE J Sel Top Quantum Electron. 1996;2:801–9. https://doi.org/10.1109/2944.577302.
Feit MD, Rubenchik AM, Kim BM, da Silva LB, Perry MD. Physical characterization of ultrashort laser pulse drilling of biological tissue. Appl Surf Sci. 1998;127–129:869–74. https://doi.org/10.1016/S0169-4332(97)00758-7.
Schwab B, Hagner D, Muller W, Lubatschowski H, Lenarz T, Heermann R. Bone ablation using ultrashort laser pulses. A new technique for middle ear surgery. Laryngorhinootologie. 2004;83:219–25. https://doi.org/10.1055/s-2004-814270.
Emigh B, An R, Hsu EM, Crawford TH, Haugen HK, Wohl GR, et al. Porcine cortical bone ablation by ultrashort pulsed laser irradiation. J Biomed Opt. 2012;17:028001. https://doi.org/10.1117/1.JBO.17.2.028001.
Jiang F, Yang X, Dai N, Lu P, Long H, Cui L. An in vitro study of femtosecond laser photodisruption in rabbit sclera. Front Optoelectron China. 2008;1:162–7. https://doi.org/10.1007/s12200-008-0022-4.
Frederickson KS, White WE, Wheeland RG, Slaughter DR. Precise ablation of skin with reduced collateral damage using the femtosecond-pulsed, terawatt titanium-sapphire laser. Arch Dermatol. 1993;129:989–93.
Mian SI, Shtein RM. Femtosecond laser-assisted corneal surgery. Curr Opin Ophthalmol. 2007;18:295–9. https://doi.org/10.1097/ICU.0b013e3281a4776c.
Farid M, Steinert RF. Femtosecond laser-assisted corneal surgery. Curr Opin Ophthalmol. 2010;21:288–92. https://doi.org/10.1097/ICU.0b013e32833a8dbc.
Coskun S, Hollanders J, Al-Hassan S, Al-Sufyan H, Al-Mayman H, Jaroudi K. Day 5 versus day 3 embryo transfer: a controlled randomized trial. Hum Reprod. 2000;15:1947–52. https://doi.org/10.1093/humrep/15.9.1947.
Bungum M, Bungum L, Humaidan P, Yding AC. Day 3 versus day 5 embryo transfer: a prospective randomized study. Reprod BioMed Online. 2003;7:98–104.
Hatirnaz S, Perkas MK. Day 3 embryo transfer versus day 5 blastocyst transfers: a prospective randomized controlled trial. Turk J Obstet Gynecol. 2017;14:82–8. https://doi.org/10.4274/tjod.99076.
Acknowledgements
The work was conducted using Unique Facility “Terawatt Femtosecond Laser Complex” in the Center for Collective Usage “Femtosecond Laser Complex” of JIHT RAS.
Funding
The reported study was funded by RFBR and Moscow city Government according to the research project no.19-32-70036.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study involving animals were in accordance with the ethical standards of the Moscow State University.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ilina, I.V., Khramova, Y.V., Filatov, M.A. et al. Femtosecond laser is effective tool for zona pellucida engraving and tagging of preimplantation mammalian embryos. J Assist Reprod Genet 36, 1251–1261 (2019). https://doi.org/10.1007/s10815-019-01424-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01424-x