Abstract
Purpose
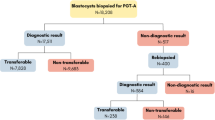
In vitro fertilization with trophectoderm embryo biopsy and pre-implantation genetic screening with comprehensive chromosomal screening (PGS-CCS) for aneuploidy is becoming increasingly more popular. Embryos are cryopreserved and implanted in a subsequent frozen thawed embryo transfer cycle (FET). No studies have investigated differences in pregnancy outcomes by timing of trophectoderm biopsy relative to stages of blastocyst development.
Methods
Retrospective study of all patients (n = 363) at a single IVF center between January 1, 2013 and December 31, 2016 undergoing single embryo transfer with PGS-CCS where embryos were cryopreserved with subsequent FET. Embryo expansion and grading was assessed both at the time of biopsy and transfer. Pregnancy rates were analyzed by embryo expansion and embryo grading.
Results
Implantation, clinical pregnancy, and live birth rates improved significantly with increased embryo expansion at the time of embryo biopsy (P < 0.001). Pregnancy loss decreased with increases in embryo expansion prior to biopsy (P < 0.001). Superior live birth rates with PGS-CCS were seen when embryos were hatching at the time of biopsy (p < 0.001). For fresh and frozen embryo transfers without PGS-CCS, embryo expansion did not affect pregnancy outcomes.
Conclusions
PGS-CCS significantly increases implantation and live birth rates only if embryos are hatching at the time of biopsy. The embryo biopsy itself on a non-hatching embryo significantly damages the embryo in ways which are not reflected in future embryo expansion. IVF labs should wait until embryos hatch before performing trophectoderm biopsy.


Similar content being viewed by others
Availability of data and material
All data is publicly available at https://osf.io/ under project “timing of PGD biopsy” under Michael Traub.
Abbreviations
- IVF:
-
In vitro fertilization
- PGS-CCS:
-
preimplantation genetic screening with complete chromosomal analysis
- FET:
-
frozen thawed embryo transfer
- eSET:
-
elective single embryo transfer
References
Kalma Y, Bar-El L, Asaf-Tisser S, Malcov M, Reches A, Hasson J, et al. Optimal timing for blastomere biopsy of 8-cell embryos for preimplantation genetic diagnosis. Hum Reprod. 2017;33:1–7. https://doi.org/10.1093/humrep/dex343.
Carson SA, Gentry WL, Smith AL, Buster JE. Trophectoderm microbiopsy in murine blastocysts: comparison of four methods. J Assist Reprod Genet. 1993;10(6):427–33.
Dokras A, Sargent IL, Gardner RL, Barlow DH. Human trophectoderm biopsy and secretion of chorionic gonadotrophin. Hum Reprod. 1991;6(10):1453–9.
Gianaroli L, Magli MC, Ferraretti AP. The in vivo and in vitro efficiency and efficacy of PGD for aneuploidy. Mol Cell Endocrinol. 2001;183(Suppl 1):S13–8.
Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–63 e1. https://doi.org/10.1016/j.fertnstert.2013.11.004.
Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod BioMed Online. 2012;24(6):614–20. https://doi.org/10.1016/j.rbmo.2012.02.009.
Harton GL, Munne S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–703. https://doi.org/10.1016/j.fertnstert.2013.07.2002.
Lopes AS, Frederickx V, Van Kerkhoven G, Campo R, Puttemans P, Gordts S. Survival, re-expansion and cell survival of human blastocysts following vitrification and warming using two vitrification systems. J Assist Reprod Genet. 2015;32(1):83–90. https://doi.org/10.1007/s10815-014-0373-2.
Li Z, Wang YA, Ledger W, Edgar DH, Sullivan EA. Clinical outcomes following cryopreservation of blastocysts by vitrification or slow freezing: a population-based cohort study. Hum Reprod. 2014;29(12):2794–801. https://doi.org/10.1093/humrep/deu246.
Sifer C. Contribution of embryo vitrification procedure to ART efficiency. Gynecol Obstet Fertil. 2014;42(10):721–4. https://doi.org/10.1016/j.gyobfe.2014.07.031.
Van Landuyt L, Van de Velde H, De Vos A, Haentjens P, Blockeel C, Tournaye H, et al. Influence of cell loss after vitrification or slow-freezing on further in vitro development and implantation of human day 3 embryos. Hum Reprod. 2013;28(11):2943–9. https://doi.org/10.1093/humrep/det356.
Pavone ME, Innes J, Hirshfeld-Cytron J, Kazer R, Zhang J. Comparing thaw survival, implantation and live birth rates from cryopreserved zygotes, embryos and blastocysts. J Human Reprod Sci. 2011;4(1):23–8. https://doi.org/10.4103/0974-1208.82356.
Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, et al. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril. 2017;107(3):723–30 e3. https://doi.org/10.1016/j.fertnstert.2016.12.022.
Karacan M, Erdem E, Usta A, Arvas A, Cebi Z, Camlibel T. Gonadotropin-releasing hormone agonist triggering with concomitant administration of low doses of human chorionic gonadotropin or a freeze-all strategy in high responders. Saudi Med J. 2017;38(6):586–91. https://doi.org/10.15537/smj.2017.6.17717.
Zech J, Brandao A, Zech M, Lugger K, Neururer S, Ulmer H, et al. Elective frozen-thawed embryo transfer (FET) in women at risk for ovarian hyperstimulation syndrome. Reprod Biol. 2017;18:46–52. https://doi.org/10.1016/j.repbio.2017.12.004.
Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome (OHSS). Hum Reprod Update. 2003;9(1):77–96.
Endo T, Honnma H, Hayashi T, Chida M, Yamazaki K, Kitajima Y, et al. Continuation of GnRH agonist administration for 1 week, after hCG injection, prevents ovarian hyperstimulation syndrome following elective cryopreservation of all pronucleate embryos. Hum Reprod. 2002;17(10):2548–51.
Martinez MC, Ruiz FJ, Garcia-Velasco JA. GnRH-agonist triggering to avoid ovarian hyperstimulation syndrome: a review of the evidence. Curr Drug Targets. 2013;14(8):843–9.
Prapas Y, Ravanos K, Petousis S, Panagiotidis Y, Papatheodorou A, Margioula-Siarkou C, et al. GnRH antagonist administered twice the day before hCG trigger combined with a step-down protocol may prevent OHSS in IVF/ICSI antagonist cycles at risk for OHSS without affecting the reproductive outcomes: a prospective randomized control trial. J Assist Reprod Genet. 2017;34(11):1537–45. https://doi.org/10.1007/s10815-017-1010-7.
Pontre JC, Ryan JP, Tan A, Hart RJ. The interval transfer of a frozen-thawed embryo is more successful than a fresh embryo transfer for women undergoing IVF with recurrent implantation failure after cleavage stage embryo biopsy. Aust N Z J Obstet Gynaecol. 2018. https://doi.org/10.1111/ajo.12798.
Roque M, Valle M, Guimaraes F, Sampaio M, Geber S. Freeze-all policy: fresh vs. frozen-thawed embryo transfer. Fertil Steril. 2015;103(5):1190–3. https://doi.org/10.1016/j.fertnstert.2015.01.045.
Adeviye Ersahin A, Acet M, Ersahin SS, Dokuzeylul GN. Frozen embryo transfer prevents the detrimental effect of high estrogen on endometrium receptivity. J Turk German Gynecological Assoc. 2017;18(1):38–42. https://doi.org/10.4274/jtgga.2016.0186.
Wu K, Zhao H, Liu H, Li M, Ma S, Li C, et al. Day 3 ET, single blastocyst transfer (SBT) or frozen-thawed embryo transfer (FET): which is preferable for high responder patients in IVF/ICSI cycles? J Assist Reprod Genet. 2014;31(3):275–8. https://doi.org/10.1007/s10815-013-0156-1.
Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril. 2013;99(2):389–92. https://doi.org/10.1016/j.fertnstert.2012.09.044.
Zhou F, Lin XN, Tong XM, Li C, Liu L, Jin XY, et al. A frozen-thawed embryo transfer program improves the embryo utilization rate. Chin Med J. 2009;122(17):1974–8.
Roy TK, Bradley CK, Bowman MC, McArthur SJ. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertil Steril. 2014;101(5):1294–301. https://doi.org/10.1016/j.fertnstert.2014.01.046.
Maheshwari A, Raja EA, Bhattacharya S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the human fertilisation and embryology authority anonymized dataset. Fertil Steril. 2016;106(7):1703–8. https://doi.org/10.1016/j.fertnstert.2016.08.047.
Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375(6):523–33. https://doi.org/10.1056/NEJMoa1513873.
Ozgur K, Berkkanoglu M, Bulut H, Humaidan P, Coetzee K. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: retrospective analysis. Fertil Steril. 2015;104(4):899–907 e3. https://doi.org/10.1016/j.fertnstert.2015.06.031.
Sun L, Chen ZH, Yin MN, Deng Y. Pregnancy and obstetric outcomes of fresh embryo transfer versus frozen-thawed embryo transfer in women below 35 years of age. Nan fang yi ke da xue xue bao = Journal of Southern Medical University. 2017;37(7):929–32.
Litzky JF, Boulet SL, Esfandiari N, Zhang Y, Kissin DM, Theiler RN, et al. Effect of frozen/thawed embryo transfer on birthweight, macrosomia, and low birthweight rates in US singleton infants. Am J Obstet Gynecol. 2018;218(4):433 e1–e10. https://doi.org/10.1016/j.ajog.2017.12.223.
Vidal M, Vellve K, Gonzalez-Comadran M, Robles A, Prat M, Torne M, et al. Perinatal outcomes in children born after fresh or frozen embryo transfer: a Catalan cohort study based on 14,262 newborns. Fertil Steril. 2017;107(4):940–7. https://doi.org/10.1016/j.fertnstert.2017.01.021.
Sha T, Yin X, Cheng W, Massey IY. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: a meta-analysis. Fertil Steril. 2018;109(2):330–42 e9. https://doi.org/10.1016/j.fertnstert.2017.10.019.
Van Heertum K, Weinerman R. Neonatal outcomes following fresh as compared to frozen/thawed embryo transfer in in vitro fertilization. Birth Defects Res. 2018;110(8):625–9. https://doi.org/10.1002/bdr2.1216.
Dahdouh EM, Balayla J, Garcia-Velasco JA. Comprehensive chromosome screening improves embryo selection: a meta-analysis. Fertil Steril. 2015;104(6):1503–12. https://doi.org/10.1016/j.fertnstert.2015.08.038.
Chen M, Wei S, Hu J, Quan S. Can comprehensive chromosome screening technology improve IVF/ICSI outcomes? A meta-analysis. PloS One. 2015;10(10):e0140779. https://doi.org/10.1371/journal.pone.0140779.
Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100(1):100–7 e1. https://doi.org/10.1016/j.fertnstert.2013.02.056.
Scott RT Jr, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100(3):697–703. https://doi.org/10.1016/j.fertnstert.2013.04.035.
Rubio C, Bellver J, Rodrigo L, Bosch E, Mercader A, Vidal C, et al. Preimplantation genetic screening using fluorescence in situ hybridization in patients with repetitive implantation failure and advanced maternal age: two randomized trials. Fertil Steril. 2013;99(5):1400–7. https://doi.org/10.1016/j.fertnstert.2012.11.041.
Keltz MD, Vega M, Sirota I, Lederman M, Moshier EL, Gonzales E, et al. Preimplantation genetic screening (PGS) with comparative genomic hybridization (CGH) following day 3 single cell blastomere biopsy markedly improves IVF outcomes while lowering multiple pregnancies and miscarriages. J Assist Reprod Genet. 2013;30(10):1333–9. https://doi.org/10.1007/s10815-013-0070-6.
Shinar S, Kornecki N, Schwartz T, Mey-Raz N, Amir H, Almog B, et al. Timing embryo biopsy for PGD - before or after cryopreservation? Gynecol Endocrinol. 2016;32(9):756–8. https://doi.org/10.1080/09513590.2016.1177010.
Linan A, Lawrenz B, El Khatib I, Bayram A, Arnanz A, Rubio C, et al. Clinical reassessment of human embryo ploidy status between cleavage and blastocyst stage by next generation sequencing. PLoS One. 2018;13(8):e0201652. https://doi.org/10.1371/journal.pone.0201652.
Cohen J, Wells D, Munne S. Removal of 2 cells from cleavage stage embryos is likely to reduce the efficacy of chromosomal tests that are used to enhance implantation rates. Fertil Steril. 2007;87(3):496–503. https://doi.org/10.1016/j.fertnstert.2006.07.1516.
Munne S, Cohen J, Simpson JL. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357(17):1769–70; author reply 70-1. https://doi.org/10.1056/NEJMc076314.
Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69(1):84–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The study was approved by the Northwell Health IRB HS16-0380. The study did not require patient consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, S., Hobeika, E., Knochenhauer, E.S. et al. Pregnancy rates after pre-implantation genetic screening for aneuploidy are only superior when trophectoderm biopsy is performed on hatching embryos. J Assist Reprod Genet 36, 621–628 (2019). https://doi.org/10.1007/s10815-019-01400-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01400-5