Abstract
Purpose
It was reported that mitochondrial DNA (mtDNA) was significantly increased in aneuploid human embryos compared to euploid embryos and was also associated with maternal age. In this study, we further established the mouse model of mtDNA quantitation in reproductive samples based on whole-genome amplification (WGA) and next-generation sequencing (NGS).
Methods
WGA followed by NGS-based mtDNA quantitation was first performed on 6 single- and 100-cell samples from a tumor-derived mouse cell line, which was exposed to ethidium bromide to reduce mtDNA content. The relative mtDNA content was normalized to nuclear DNA. This method was then applied to mouse reproductive samples, including 40 pairs of oocytes and polar bodies from 8 CD-1 female mice of advanced reproductive age and 171 blastocysts derived via in vitro maturation (IVM) or in vivo maturation (IVO) from young (6–9 weeks) and reproductively aged (13.5 months) female CF-1 mice.
Results
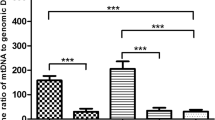
Exposure to ethidium bromide for 3 and 6 days decreased mtDNA levels in both the single- and 100-cell samples as expected. Results demonstrated that the first polar body contained an average of 0.9% of mtDNA relative to oocytes. Compared to the cells in blastocysts, oocytes contained about 180 times as much mtDNA per cell. mtDNA levels were compared among blastocysts from reproductively young and old female mice that had either been produced by IVM or IVO. Cells in blastocysts from younger mice contained significantly lower amounts of mtDNA compared to aged mice (P < 0.0001). Cells in blastocysts produced via IVO had higher mtDNA content than IVM-derived blastocysts (P = 0.0001). Cells in aneuploid blastocysts were found to have significantly higher (1.74-fold) levels of mtDNA compared to euploid blastocysts (P = 0.0006).
Conclusion
A reliable method for assessing mtDNA content in mouse gametes and embryos was established. Relative mtDNA levels were elevated in aneuploid embryos relative to euploid embryos, were higher in blastocysts from reproductively old mice relative to young mice, and were lower in embryos derived from IVM compared to IVO.



Similar content being viewed by others
References
Scott RT, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013;100:697–703.
Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93.
Voet D, Voet JG, Pratt CW. Fundamentals of biochemistry. 2nd ed. Hoboken: Wiley; 2006.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13.
Huang K, Manton KG. The role of oxidative damage in mitochondria during aging: a review. Front Biosci. 2004;9:1100–17.
Wiesner RJ, Rüegg JC, Morano I. Counting target molecules by exponential polymerase chain reaction: copy number of mitochondrial DNA in rat tissues. Biochem Biophys Res Commun. 1992;183:553–9.
Su J, Tao X, Baglione G, Treff NR, Scott RT. Mitochondrial DNA is significantly increased in aneuploid human embryos. Fertil Steril. 2010;94:S88–9.
Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11:e1005241.
Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertil Steril. 2015;104:534–41.
Treff NR, Zhan Y, Tao X, Olcha M, Han M, Rajchel J, et al. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum Reprod. 2017;32(4):954–62.
Treff NR, Krisher RL, Tao X, Garnsey H, Bohrer C, Silva E, et al. Next generation sequencing-based comprehensive chromosome screening in mouse polar bodies, oocytes, and embryos. Biol Reprod. 2016;94:76.
Jones KT, Lane SI. Molecular causes of aneuploidy in mammalian eggs. Development. 2013;140:3719–30.
Merriman JA, Jennings PC, McLaughlin EA, Jones KT. Effect of aging on superovulation efficiency, aneuploidy rates, and sisterchromatid cohesion in mice aged up to 15 months. Biol Reprod. 2012;86:49.
Kuliev A, Zlatopolsky Z, Kirillova I, Spivakova J, Cieslak JJ. Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod BioMed Online. 2011;22:2–8.
MacLennan M, Crichton JH, Playfoot CJ, Adams IR. Oocyte development, meiosis and aneuploidy. Semin Cell Dev Biol. 2015;45:68–76.
Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–9.
Downs SM. Regulation of the G2/M transition in rodent oocytes. Mol Reprod Dev. 2010;77:566–85.
Cotterill M, Harris SE, Collado Fernandez E, Lu J, Huntriss JD, Campbell BK, et al. The activity and copy number of mitochondrial DNA in ovine oocytes throughout oogenesis in vivo and during oocyte maturation in vitro. Mol Hum Reprod. 2013;19:444–50.
Nacarelli T, Azar A, Sell C. Inhibition of mTOR prevents ROS production initiated by ethidium bromide-induced mitochondrial DNA depletion. Front Endocrinol (Lausanne). 2014;5:122.
Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, et al. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil Steril. 2017;107:34–42.
Treff NR, Su J, Tao X, Levy B, Scott RT Jr. Accurate single cell 24 chromosome aneuploidy screening using whole genome amplification and single nucleotide polymorphism microarrays. Fertil Steril. 2010;94:2017–21.
Hornick JE, Duncan FE, Sun M, Kawamura R, Marko JF, Woodruff TK. Age-associated alterations in the micromechanical properties of chromosomes in the mammalian egg. J Assist Reprod Genet. 2015;32:765–9.
Silva E, Greene AF, Strauss K, Herrick JR, Schoolcraft WB, Krisher RL. Antioxidant supplementation during in vitro culture improves mitochondrial function and development of embryos from aged female mice. Reprod Fertil Dev. 2015;27(6):975–83.
Yun Y, Lane SI, Jones KT. Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Development. 2014;141:199–208.
Piko L, Taylor KD. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol. 1987;123:364–74.
Thundathil J, Filion F, Smith LC. Molecular control of mitochondrial function in preimplantation mouse embryos. Mol Reprod Dev. 2005;71:405–13.
St John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update. 2010;16:488–509.
Dalton CM, Carroll J. Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte. J Cell Sci. 2013;126:2955–64.
Wang Q, Ratchford AM, Chi MM, Schoeller E, Frolova A, Schedl T, et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23:1603–12.
Leese HJ, Baumann CG, Brison DR, McEvoy TG, Sturmey RG. Metabolism of the viable mammalian embryo: quietness revisited. Mol Hum Reprod. 2008;14:667–72.
Leese HJ. Metabolism of the preimplantation embryo: 40 years on. Reproduction. 2012;143:417–27.
Rambags BP, van Boxtel DC, Tharasanit T, Lenstra JA, Colenbrander B, Stout TA. Advancing maternal age predisposes to mitochondrial damage and loss during maturation of equine oocytes in vitro. Theriogenology. 2014;81:959–65.
Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, et al. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev. 2011;23:424–32.
Wang LY, Wang DH, Zou XY, Xu CM. Mitochondrial functions on oocytes and preimplantation embryos. J Zhejiang Univ Sci B. 2009;10:483–92.
Tolkovsky AM, Xue L, Fletcher GC, Borutaite V. Mitochondrial disappearance from cells: a clue to the role of autophagy in programmed cell death and disease? Biochimie. 2002;84:233–40.
Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5.
Sanfins A, Plancha CE, Albertini DF. Pre-implantation developmental potential from in vivo and in vitro matured mouse oocytes: a cytoskeletal perspective on oocyte quality. J Assist Reprod Genet. 2015;32:127–36.
Zhou C-J, Wu S-N, Shen J-P, Wang D-H, Kong X-W, Lu A, et al. The beneficial effects of cumulus cells and oocyte-cumulus cell gap junctions depends on oocyte maturation and fertilization methods in mice. Peer J. 2016;4:e1761.
Reynier P, May-Panloup P, Chrétien MF, Morgan CJ, Jean M, Savagner F, et al. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7:425–9.
Fragouli E, McCaffrey C, Ravichandran K, Spath K, Grifo J, Munné S, et al. Clinical implications of mitochondrial DNA quantification on pregnancy outcomes: a blinded prospective non-selection study. Hum Reprod. 2017; https://doi.org/10.1093/humrep/dex292.
Ravichandran K, McCaffrey C, Grifo J, Morales A, Perloe M, Munne S, et al. Mitochondrial DNA quantification as a tool for embryo viability assessment: retrospective analysis of data from single euploid blastocyst transfers. Hum Reprod. 2017;32(6):1282–92.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were approved by the Institutional Animal Care and Use Committee (Northwestern University) or Colorado Center for Reproductive Medicine Ethics in Research Committee and were carried out in accordance with National Institutes of Health Guidelines and the Society for the Study of Reproduction’s specific guidelines and standards.
Rights and permissions
About this article
Cite this article
Tao, X., Landis, J.N., Krisher, R.L. et al. Mitochondrial DNA content is associated with ploidy status, maternal age, and oocyte maturation methods in mouse blastocysts. J Assist Reprod Genet 34, 1587–1594 (2017). https://doi.org/10.1007/s10815-017-1070-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-1070-8