Abstract
Purpose
The purpose of the present study is to test whether metformin, aspirin, or diet supplement (DS) BioResponse-3,3′-Diindolylmethane (BR-DIM) can induce AMP-activated protein kinase (AMPK)-dependent potency loss in cultured embryos and whether metformin (Met) + Aspirin (Asa) or BR-DIM causes an AMPK-dependent decrease in embryonic development.
Methods
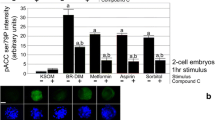
The methods used were as follows: culture post-thaw mouse zygotes to the two-cell embryo stage and test effects after 1-h AMPK agonists’ (e.g., Met, Asa, BR-DIM, control hyperosmotic stress) exposure on AMPK-dependent loss of Oct4 and/or Rex1 nuclear potency factors, confirm AMPK dependence by reversing potency loss in two-cell-stage embryos with AMPK inhibitor compound C (CC), test whether Met + Asa (i.e., co-added) or DS BR-DIM decreases development of two-cell to blastocyst stage in an AMPK-dependent (CC-sensitive) manner, and evaluate the level of Rex1 and Oct4 nuclear fluorescence in two-cell-stage embryos and rate of two-cell-stage embryo development to blastocysts.
Result(s)
Met, Asa, BR-DIM, or hyperosmotic sorbitol stress induces rapid ~50–85 % Rex1 and/or Oct4 protein loss in two-cell embryos. This loss is ~60–90 % reversible by co-culture with AMPK inhibitor CC. Embryo development from two-cell to blastocyst stage is decreased in culture with either Met + Asa or BR-DIM, and this is either >90 or ~60 % reversible with CC, respectively.
Conclusion
These experimental designs here showed that Met-, Asa-, BR-DIM-, or sorbitol stress-induced rapid potency loss in two-cell embryos is AMPK dependent as suggested by inhibition of Rex1 and/or Oct4 protein loss with an AMPK inhibitor. The DS BR-DIM or fertility drugs (e.g., Met + Asa) that are used to enhance maternal metabolism to support fertility can also chronically slow embryo growth and block development in an AMPK-dependent manner.





Similar content being viewed by others
References
Duranteau L, Lefevre P, Jeandidier N, Simon T, Christin-Maitre S. Should physicians prescribe metformin to women with polycystic ovary syndrome PCOS? Ann Endocrinol (Paris). 2010;71:25–7.
Palomba S, Pasquali R, Orio Jr F, Nestler JE. Clomiphene citrate, metformin or both as first-step approach in treating anovulatory infertility in patients with polycystic ovary syndrome (PCOS): a systematic review of head-to-head randomized controlled studies and meta-analysis. Clin Endocrinol (Oxf). 2009;70:311–21.
Sinawat S, Buppasiri P, Lumbiganon P, Pattanittum P. Long versus short course treatment with metformin and clomiphene citrate for ovulation induction in women with PCOS. Cochrane Database Syst Rev 2008:CD006226.
Jamal A, Milani F, Al-Yasin A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran J Reprod Med. 2012;10:265–70.
de Oliveira Baraldi C, Lanchote VL, de Jesus Antunes N, de Jesus Ponte Carvalho TM, Dantas Moises EC, Duarte G, et al. Metformin pharmacokinetics in nondiabetic pregnant women with polycystic ovary syndrome. Eur J Clin Pharmacol. 2011;67:1027–33.
Vause TD, Cheung AP, Sierra S, Claman P, Graham J, Guillemin JA, et al. Ovulation induction in polycystic ovary syndrome. J Obstet Gynaecol Can. 2010;32:495–502.
Jungheim ES, Odibo AO. Fertility treatment in women with polycystic ovary syndrome: a decision analysis of different oral ovulation induction agents. Fertil Steril. 2010;94:2659–64.
Genazzani AD, Ricchieri F, Lanzoni C. Use of metformin in the treatment of polycystic ovary syndrome. Womens Health (Lond Engl). 2010;6:577–93.
Palomba S, Falbo A, Russo T, Orio F, Tollino A, Zullo F. Role of metformin in patients with polycystic ovary syndrome: the state of the art. Minerva Ginecol. 2008;60:77–82.
Escobar-Morreale HF. Polycystic ovary syndrome: treatment strategies and management. Expert Opin Pharmacother. 2008;9:2995–3008.
Moll E, van der Veen F, van Wely M. The role of metformin in polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2007;13:527–37.
Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–66.
Cheang KI, Sharma ST, Nestler JE. Is metformin a primary ovulatory agent in patients with polycystic ovary syndrome? Gynecol Endocrinol. 2006;22:595–604.
Carrington B, Sacks G, Regan L. Recurrent miscarriage: pathophysiology and outcome. Curr Opin Obstet Gynecol. 2005;17:591–7.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74.
Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22.
Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–36.
Del Priore G, Gudipudi DK, Montemarano N, Restivo AM, Malanowska-Stega J, Arslan AA. Oral diindolylmethane (DIM): pilot evaluation of a nonsurgical treatment for cervical dysplasia. Gynecol Oncol. 2010;116:464–7.
Ribaux P, Irion O, Cohen M. An active product of cruciferous vegetables, 3,3'-diindolylmethane, inhibits invasive properties of extravillous cytotrophoblastic cells. Neuro Endocrinol Lett. 2012;33:133–7.
Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–8.
Chen D, Banerjee S, Cui QC, Kong D, Sarkar FH, Dou QP. Activation of AMP-activated protein kinase by 3,3'-diindolylmethane (DIM) is associated with human prostate cancer cell death in vitro and in vivo. PLoS ONE. 2012;7, e47186.
LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006;74:585–92.
Louden ED, Luzzo KM, Jimenez PT, Chi T, Chi M, Moley KH. TallyHO obese female mice experience poor reproductive outcomes and abnormal blastocyst metabolism that is reversed by metformin. Reprod Fertil Dev. 2014;27:31–9.
Louden E, Chi MM, Moley KH. Crosstalk between the AMP-activated kinase and insulin signaling pathways rescues murine blastocyst cells from insulin resistance. Reproduction. 2008;136:335–44.
Ratchford AM, Chang AS, Chi MM, Sheridan R, Moley KH. Maternal diabetes adversely affects AMP-activated protein kinase activity and cellular metabolism in murine oocytes. Am J Physiol Endocrinol Metab. 2007;293:E1198–206.
Solano ME, Elia E, Luchetti CG, Sander V, Di Girolamo G, Gonzalez C, et al. Metformin prevents embryonic resorption induced by hyperandrogenisation with dehydroepiandrosterone in mice. Reprod Fertil Dev. 2006;18:533–44.
Larosa C, Downs SM. Meiotic induction by heat stress in mouse oocytes: involvement of AMP-activated protein kinase and MAPK family members. Biol Reprod. 2007;76:476–86.
Chen J, Hudson E, Chi MM, Chang AS, Moley KH, Hardie DG et al. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev Biol 2006.
Rappolee DA. Impact of transient stress and stress enzymes on development. Dev Biol. 2007;304:1–8.
Puscheck EE, Awonuga AO, Yang Y, Jiang Z, Rappolee DA. Molecular biology of the stress response in the early embryo and its stem cells. Adv Exp Med Biol. 2015;843:77–128.
Mansouri L, Xie Y, Rappolee DA. Adaptive and pathogenic responses to stress by stem cells during development. Cells. 2012;1:1197–224.
Xie Y, Awonuga AO, Zhou S, Puscheck EE, Rappolee DA. Interpreting the stress response of early mammalian embryos and their stem cells. Int Rev Cell Mol Biol. 2011;287:43–95.
Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–83.
Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10.
Hardie DG. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology. 2006;131:973. author reply 4-5.
Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32.
Yue W, Wang T, Zachariah E, Lin Y, Yang CS, Xu Q, et al. Transcriptomic analysis of pancreatic cancer cells in response to metformin and aspirin: an implication of synergy. Sci Rep. 2015;5:13390.
Yue W, Yang CS, DiPaola RS, Tan XL. Repurposing of metformin and aspirin by targeting AMPK-mTOR and inflammation for pancreatic cancer prevention and treatment. Cancer Prev Res. 2014;7:388–97.
Ford RJ, Fullerton MD, Pinkosky SL, Day EA, Scott JW, Oakhill JS, et al. Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem J. 2015;468:125–32.
Downs SM, Hudson ER, Hardie DG. A potential role for AMP-activated protein kinase in meiotic induction in mouse oocytes. Dev Biol. 2002;245:200–12.
Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, et al. Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction. 2010;140:921–30.
Chae HD, Lee MR, Broxmeyer HE. 5-Aminoimidazole-4-carboxyamide ribonucleoside induces G(1)/S arrest and Nanog downregulation via p53 and enhances erythroid differentiation. Stem Cells. 2012;30:140–9.
Vazquez-Martin A, Vellon L, Quiros PM, Cufi S, Ruiz de Galarreta E, Oliveras-Ferraros C, et al. Activation of AMP-activated protein kinase (AMPK) provides a metabolic barrier to reprogramming somatic cells into stem cells. Cell Cycle. 2012;11:974–89.
Yang Y, Jiang Z, Bolnick A, Dai J, Puscheck E, Rappolee D. Departure from optimal 2% O2 level for TSC potency and proliferation leads to most rapid increases in AMPK activity. Submitted to Placenta 2015.
Xie Y, Awonuga A, Liu J, Rings E, Puscheck EE, Rappolee DA. Stress induces AMPK-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells [corrected]. Stem Cells Dev. 2013;22:1564–75.
Xie Y, Abdallah ME, Awonuga AO, Slater JA, Puscheck EE, Rappolee DA. Benzo(a)pyrene causes PRKAA1/2-dependent ID2 loss in trophoblast stem cells. Mol Reprod Dev. 2010;77:533–9.
Guo Y, Mantel C, Hromas RA, Broxmeyer HE. Oct-4 is critical for survival/antiapoptosis of murine embryonic stem cells subjected to stress: effects associated with Stat3/survivin. Stem Cells. 2008;26:30–4.
Kang J, Shakya A, Tantin D. Stem cells, stress, metabolism and cancer: a drama in two Octs. Trends Biochem Sci. 2009;34:491–9.
Kang J, Gemberling M, Nakamura M, Whitby FG, Handa H, Fairbrother WG, et al. A general mechanism for transcription regulation by Oct1 and Oct4 in response to genotoxic and oxidative stress. Genes Dev. 2009;23:208–22.
Frum T, Halbisen MA, Wang C, Amiri H, Robson P, Ralston A. Oct4 cell-autonomously promotes primitive endoderm development in the mouse blastocyst. Dev Cell. 2013;25:610–22.
Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91.
Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6.
Hogan B, Beddington R, Constantini F, Lacy B. Manipulating the mouse embryo: a laboratory manual. 3rd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory; 2002.
Wang Y, Puscheck EE, Lewis JJ, Trostinskaia AB, Wang F, Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril. 2005;83 Suppl 1:1144–54.
Zhong W, Xie Y, Wang Y, Lewis J, Trostinskaia A, Wang F, et al. Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci. 2007;14:534–47.
Xie Y, Zhong W, Wang Y, Trostinskaia A, Wang F, Puscheck EE, et al. Using hyperosmolar stress to measure biologic and stress-activated protein kinase responses in preimplantation embryos. Mol Hum Reprod. 2007;13:473–81.
An Y, Sun Z, Li L, Zhang Y, Ji H. Relationship between psychological stress and reproductive outcome in women undergoing in vitro fertilization treatment: psychological and neurohormonal assessment. J Assist Reprod Genet. 2013;30:35–41.
Lee HY, Wei D, Loeken MR. Lack of metformin effect on mouse embryo AMPK activity: implications for metformin treatment during pregnancy. Diabetes Metab Res Rev. 2014;30:23–30.
Wu Y, Viana M, Thirumangalathu S, Loeken MR. AMP-activated protein kinase mediates effects of oxidative stress on embryo gene expression in a mouse model of diabetic embryopathy. Diabetologia. 2012;55:245–54.
Paltsev M, Kiselev V, Muyzhnek E, Drukh V, Kuznetsov I, Pchelintseva O. Comparative preclinical pharmacokinetics study of 3,3'-diindolylmethane formulations: is personalized treatment and targeted chemoprevention in the horizon? EPMA J. 2013;4:25.
Ross-Lee LM, Elms MJ, Cham BE, Bochner F, Bunce IH, Eadie MJ. Plasma levels of aspirin following effervescent and enteric coated tablets, and their effect on platelet function. Eur J Clin Pharmacol. 1982;23:545–51.
Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–6.
Yang Y, Arenas-Hernandez M, Gomez-Lopez N, Dai J, Puscheck E, Rappolee D. Hypoxic stress forces large, irreversible trophoblast stem cell differentiation. Biol Reprod. 2016;Submitted.
Li Q, Gomez-Lopez N, Drewlo S, Sanchez-Rodriquez E, Dai J, Puscheck EE et al. Development and validation of a Rex1-RFP potency activity reporter assay that quantifies stress-forced potency loss in mouse embryonic stem cells. Stem Cells Dev 2015.
Slater JA, Zhou S, Puscheck EE, Rappolee DA. Stress-induced enzyme activation primes murine embryonic stem cells to differentiate toward the first extraembryonic lineage. Stem Cells Dev. 2014;23:3049–64.
McRae AC, Church RB. Cytoplasmic projections of trophectoderm distinguish implanting from preimplanting and implantation-delayed mouse blastocytes. J Reprod Fertil. 1990;88:31–40.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Freeze-all at the blastocyst or bipronuclear stage: a randomized clinical trial. Fertil Steril. 2015;104:1138–44.
Rice S, Elia A, Jawad Z, Pellatt L, Mason HD. Metformin inhibits follicle-stimulating hormone (FSH) action in human granulosa cells: relevance to polycystic ovary syndrome. J Clin Endocrinol Metab. 2013;98:E1491–500.
Kovacs G. How to improve your ART success rates: an evidence-based review of adjuncts to IVF. Cambridge. New York: Cambridge University Press; 2011.
Palomba S, Orio Jr F, Russo T, Falbo A, Cascella T, Colao A, et al. Is ovulation induction still a therapeutic problem in patients with polycystic ovary syndrome? J Endocrinol Invest. 2004;27:796–805.
Kashyap S, Wells GA, Rosenwaks Z. Insulin-sensitizing agents as primary therapy for patients with polycystic ovarian syndrome. Hum Reprod. 2004;19:2474–83.
Ben-Haroush A, Yogev Y, Fisch B. Insulin resistance and metformin in polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2004;115:125–33.
Barbieri RL. Metformin for the treatment of polycystic ovary syndrome. Obstet Gynecol. 2003;101:785–93.
Nestler JE, Stovall D, Akhter N, Iuorno MJ, Jakubowicz DJ. Strategies for the use of insulin-sensitizing drugs to treat infertility in women with polycystic ovary syndrome. Fertil Steril. 2002;77:209–15.
Phipps WR. Polycystic ovary syndrome and ovulation induction. Obstet Gynecol Clin N Am. 2001;28:165–82.
Taylor R, Marsden PJ. Insulin sensitivity and fertility. Hum Fertil (Camb). 2000;3:65–9.
Goldenberg N, Glueck CJ. Is pharmacogenomics our future? Metformin, ovulation and polymorphism of the STK11 gene in polycystic ovary syndrome. Pharmacogenomics. 2008;9:1163–5.
Chen J, Hudson E, Chi MM, Chang AS, Moley KH, Hardie DG, et al. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev Biol. 2006;291:227–38.
Patel Y, Kim H, Rappolee DA. A role for hepatocyte growth factor during early postimplantation growth of the placental lineage in mice. Biol Reprod. 2000;62:904–12.
Awonuga AO, Zhong W, Abdallah ME, Slater JA, Zhou SC, Xie YF, et al. Eomesodermin, HAND1, and CSH1 proteins are induced by cellular stress in a stress-activated protein kinase-dependent manner. Mol Reprod Dev. 2011;78:519–28.
Li Q, Louden E, Dai J, Furcron A, Gomez-Lopez N, Drewlo S et al. Stress forces first lineage differentiation of mouse ESCs, validation of a high throughput screen for toxicant stress. Development 2016;manuscript in preparation.
Li Q, Gomez-Lopez N, Drewlo S, Sanchez-Rodriguez E, Dai J, Puscheck EE, et al. Development and validation of a Rex1-RFP potency activity reporter assay that quantifies stress-forced potency loss in mouse embryonic stem cells. Stem Cells Dev. 2016;25:320–8.
Goolam M, Scialdone A, Graham SJ, Macaulay IC, Jedrusik A, Hupalowska A, et al. Heterogeneity in Oct4 and Sox2 targets biases cell fate in 4-cell mouse embryos. Cell. 2016;165:61–74.
Lutwak-Mann C, Laser H. Bicarbonate content of the blastocyst fluid and carbonic anhydrase in the pregnant rabbit uterus. Nature. 1954;173:268–9.
Lutwak-Mann C, Hay MF. Effect on the early embryo of agents administered to the mother. Br Med J. 1962;2:944–6.
Fabro S. Penetration of chemicals into the oocyte, uterine fluid, and preimplantation blastocyst. Environ Health Perspect. 1978;24:25–9.
Fabro S, McLachlan JA, Dames NM. Chemical exposure of embryos during the preimplantation stages of pregnancy: mortality rate and intrauterine development. Am J Obstet Gynecol. 1984;148:929–38.
Nielsen GL, Sorensen HT, Larsen H, Pedersen L. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ. 2001;322:266–70.
Cerletti C, Bonati M, del Maschio A, Galletti F, Dejana E, Tognoni G, et al. Plasma levels of salicylate and aspirin in healthy volunteers: relevance to drug interaction on platelet function. J Lab Clin Med. 1984;103:869–77.
Ying Y, Cai YX, Lou YJ. Effects of blastocyst deficiencies induced by aspirin treatment during preimplantation period in rats on development of embryos after implantation. Yao Xue Xue Bao. 1996;31:416–9.
Ying Y, Lou YJ. Effects of preimplantation treatment with aspirin and acetaminophen on blastocyst and fetus in rats. Zhongguo Yao Li Xue Bao. 1993;14:369–72.
Vanky E, Zahlsen K, Spigset O, Carlsen SM. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril. 2005;83:1575–8.
Enders AC. Trophoblast-uterine interactions in the first days of implantation: models for the study of implantation events in the human. Semin Reprod Med. 2000;18:255–63.
Enders AC, Lantz KC, Peterson PE, Hendrickx AG. From blastocyst to placenta: the morphology of implantation in the baboon. Hum Reprod Update. 1997;3:561–73.
Bedaiwy MA, Miller KF, Goldberg JM, Nelson D, Falcone T. Effect of metformin on mouse embryo development. Fertil Steril. 2001;76:1078–9.
Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–50.
Caille G, Lacasse Y, Raymond M, Landriault H, Perrotta M, Picirilli G, et al. Bioavailability of metformin in tablet form using a new high pressure liquid chromatography assay method. Biopharm Drug Dispos. 1993;14:257–63.
Levy G. Comparative pharmacokinetics of aspirin and acetaminophen. Arch Intern Med. 1981;141:279–81.
Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10:5233–41.
Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev. 2007;74:1287–94.
Zhang H, Legro RS, Zhang J, Zhang L, Chen X, Huang H, et al. Decision trees for identifying predictors of treatment effectiveness in clinical trials and its application to ovulation in a study of women with polycystic ovary syndrome. Hum Reprod. 2010;25:2612–21.
Palomba S, Falbo A, Russo T, Orio F, Tolino A, Zullo F. Systemic and local effects of metformin administration in patients with polycystic ovary syndrome (PCOS): relationship to the ovulatory response. Hum Reprod. 2010;25:1005–13.
O'Brien AJ, Villani LA, Broadfield LA, Houde VP, Galic S, Blandino G, et al. Salicylate activates AMPK and synergizes with metformin to reduce the survival of prostate and lung cancer cells ex vivo through inhibition of de novo lipogenesis. Biochem J. 2015;469:177–87.
Xie Y, Zhou S, Jiang Z, Dai J, Puscheck EE, Lee I, et al. Hypoxic stress induces, but cannot sustain trophoblast stem cell differentiation to labyrinthine placenta due to mitochondrial insufficiency. Stem Cell Res. 2014;13:478–91.
Zhou S, Xie Y, Puscheck EE, Rappolee DA. Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta. 2011;32:475–81.
Abdulhasan M, Yang Y, Dai J, Folger J, Cibelli J, Shubber A et al. CoQ10 improves bovine oocyte IVM, increases ATP and potency factor levels while maintaining decreased AMPK activity and stress marker levels. Biol Reprod. 2015;To be submitted.
Lee MT, Bonneau AR, Takacs CM, Bazzini AA, DiVito KR, Fleming ES, et al. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature. 2013;503:360–4.
Leichsenring M, Maes J, Mossner R, Driever W, Onichtchouk D. Pou5f1 transcription factor controls zygotic gene activation in vertebrates. Science. 2013;341:1005–9.
Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–29.
Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, et al. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–102.
Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell. 2006;125:301–13.
Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature. 2006;441:349–53.
Wu G, Gentile L, Fuchikami T, Sutter J, Psathaki K, Esteves TC, et al. Initiation of trophectoderm lineage specification in mouse embryos is independent of Cdx2. Development. 2010;137:4159–69.
Ortega I, Wong DH, Villanueva JA, Cress AB, Sokalska A, Stanley SD, et al. Effects of resveratrol on growth and function of rat ovarian granulosa cells. Fertil Steril. 2012;98:1563–73.
Moraloglu O, Engin-Ustun Y, Tonguc E, Var T, Tapisiz OL, Ergun H, et al. The effect of resveratrol on blood pressure in a rat model of preeclampsia. J Matern Fetal Neonatal Med. 2012;25:845–8.
Bourque SL, Dolinsky VW, Dyck JR, Davidge ST. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta. 2012;33:449–52.
Wong DH, Villanueva JA, Cress AB, Sokalska A, Ortega I, Duleba AJ. Resveratrol inhibits the mevalonate pathway and potentiates the antiproliferative effects of simvastatin in rat theca-interstitial cells. Fertil Steril. 2011;96:1252–8.
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42.
Murase T, Misawa K, Haramizu S, Minegishi Y, Hase T. Nootkatone, a characteristic constituent of grapefruit, stimulates energy metabolism and prevents diet-induced obesity by activating AMPK. Am J Physiol Endocrinol Metab. 2010;299:E266–75.
Han CY, Ki SH, Kim YW, Noh K, Leeda Y, Kang B, et al. Ajoene, a stable garlic by-product, inhibits high fat diet-induced hepatic steatosis and oxidative injury through LKB1-dependent AMPK activation. Antioxid Redox Signal. 2011;14:187–202.
Chinnakannu K, Chen D, Li Y, Wang Z, Dou QP, Reddy GP, et al. Cell cycle-dependent effects of 3,3'-diindolylmethane on proliferation and apoptosis of prostate cancer cells. J Cell Physiol. 2009;219:94–9.
Secor E, Hardie G, K M, Froment P, Louden E, Bonick A et al. AMPK agonists in diet supplements and pharma mediate wide-ranging somatic and reproductive effects. . eCAM 2016;Submitted.
Yang L, Sha H, Davisson RL, Qi L. Phenformin activates the unfolded protein response in an AMP-activated protein kinase (AMPK)-dependent manner. J Biol Chem. 2013;288:13631–8.
Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–7.
Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–908.
Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–65.
Acknowledgments
Thanks to Jose Cibelli, Michael Diamond, and Erica Louden for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
DAR and EEP from the Office of the Vice President for Research at Wayne State University, from the REI fellows’ fund (AB), and from the funding of the Mary Iacobell and Kamran Moghissi Endowed Chairs.
Additional information
Capsule
Drugs metformin and aspirin and diet supplement BR-DIM cause AMPK-dependent potency loss and decrease embryonic development from two-cell to blastocyst stage.
Rights and permissions
About this article
Cite this article
Bolnick, A., Abdulhasan, M., Kilburn, B. et al. Commonly used fertility drugs, a diet supplement, and stress force AMPK-dependent block of stemness and development in cultured mammalian embryos. J Assist Reprod Genet 33, 1027–1039 (2016). https://doi.org/10.1007/s10815-016-0735-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0735-z